Mclean/Shutterstock.com
Social distancing and lockdown measures put in place across the UK to limit the spread of COVID-19 have also led to a huge drop in the levels of influenza.
However, while we continue to battle the COVID-19 pandemic, it is important not to become complacent about the risks posed by the yearly emergence of new strains of flu. The virus’s ability to mutate rapidly means that when a new strain appears, immunity is low, protection from seasonal vaccines is limited and the risk of a pandemic is high.
The 1918 Spanish flu infected 500 million people — about a third of the world’s population at that time — and killed an estimated 50 million, and the most recent flu pandemic in 2009 is estimated to have infected around 24% of the global population and killed over 284,000 in its first year[1,2].
Looking at these data, it is clear that developing a universal flu vaccine to protect against multiple flu strains is more important than ever.
An arms race
The challenge with the flu virus lies in its ability to alter its surface proteins — the parts of the virus the human immune system usually targets first, which enables it to continue reinfecting the same host population over time.
“It is engaged in a kind of evolutionary process … that one could describe as an arms race,” says Sunetra Gupta, a theoretical epidemiologist and vaccine developer from the University of Oxford.
The flu virus exists in many types and subtypes: both influenza A and B can cause epidemic seasonal infections. Influenza B is typically only found in humans, but influenza A subtypes are found in many other species, particularly in birds, and if these subtypes transfer to humans the risk of a pandemic increases.
You have to race to make the vaccine
Sunetra Gupta
We are also battling against what immunologists call ‘antigenic drift’ within current flu subtypes, owing to rapid changes in the surface glycoprotein haemagglutinin (HA).
Influenza viruses are named after the 18 possible HA subtypes (which can be split into two broadly similar groups) and the 11 different subtypes of another surface protein, neuraminadase (NA). Subtypes found in humans are usually H1N1 or H3N2 but there have also been a handful of deadly outbreaks of H5N1 and H7N9 influenzas.
It is changes to the HA protein, which occur even in familiar circulating subtypes, which necessitate updating flu vaccines every year.
“You have to race to make the vaccine,” explains Gupta: “And very often there is a mismatch.”
This means that current seasonal flu vaccines have, on average, an efficacy of only 40%. There have been some industry advances, such as the new quadrivalent vaccine, which is designed to protect against four different flu viruses; and newer cell-based vaccines. However, for a long time, improving the flu vaccine was not a priority.
“Industry does not make huge amounts of money out of flu vaccines … there’s no blockbusting in this,” explains John McCauley, director of the Worldwide Influenza Centre at the Francis Crick Institute in London.
However, according to Florian Krammer, a microbiologist at the Icahn School of Medicine at Mount Sinai, New York, things started to change in 2009 after it was discovered that some of the antibodies humans make in response to influenza can neutralise multiple flu subtypes.
“That really catalysed it because that gave us a hope that we can do this with the human immune system,” he says.
By 2018, the United States National Institute for Allergy and Infectious Diseases had taken up the challenge and released a strategic plan for a universal flu vaccine and funded a US$51m collaborative network to drive its development[3,4].
Some researchers refer to “more broadly protective” vaccines, indicating a vaccine that may protect against the antigenic drift of existing strains and provide broader coverage to flu subtypes already in circulation.
A truly universal vaccine would also protect against any new subtypes that may emerge from the animal kingdom — sometimes called an ‘antigenic shift’ — which is much more difficult.
You will never demonstrate universality because you don’t know what’s coming next year
Olga Pleguezuelos
Also, according to Olga Pleguezuelos, chief scientific officer at the UK biotechnology company ConserV Bioscience: “You will never demonstrate universality because you don’t know what’s coming next year.”
In 2020, US vaccine development company Novavax started a phase III trial for its quadrivalent NanoFlu vaccine, which — although not universal — showed “significant improvements against four drifted H3N2 strains”.
However, the creation of broader vaccines is hugely challenging and several attempts have failed.
In January 2020, Oxford-based biotechnology company Vaccitech announced that phase IIb data for its universal flu vaccine — VTP-100 — had shown it had not achieved reductions in infections compared with the seasonal vaccine and that it would discontinue the trial.
Then, after 15 years of research, Israeli biotech BiondVax Pharmaceuticals announced in October 2020 its phase III study of universal flu vaccine — M-001 — showed no significant protection above placebo.
Universal strategies
One of the most promising approaches to developing a universal vaccine currently involves stimulating immunity against the parts of the mushroom-shaped HA protein that remain unchanged across strains — specifically the ‘stalk’ of the mushroom rather than the ‘head’ (see Figure).
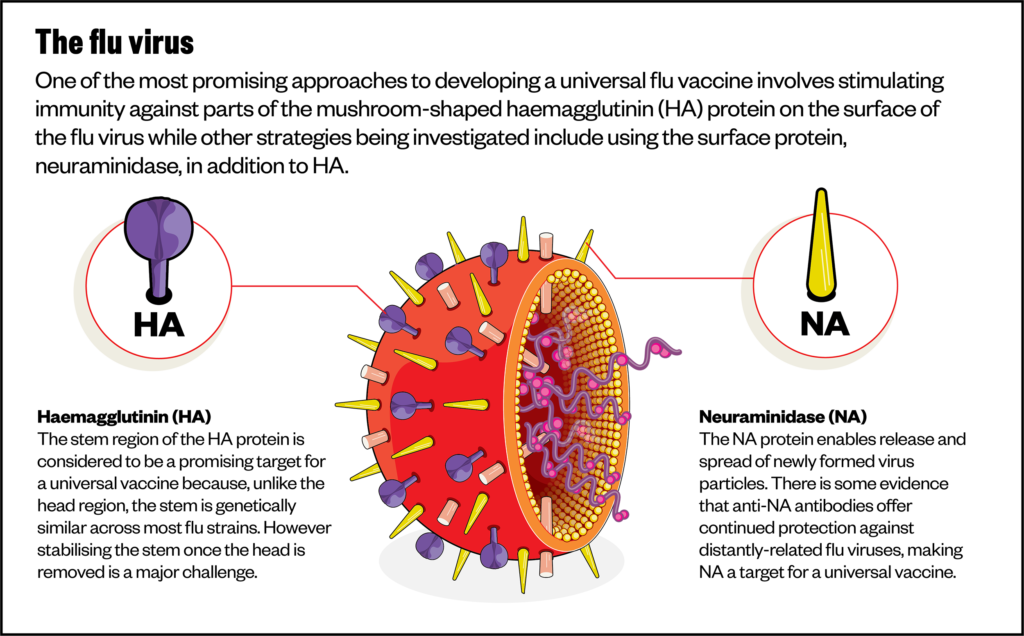
To do this, Krammer and his team made new versions of HA by combining parts from different subtypes (chimeras). Specifically, they engineered proteins made from the heads of ‘exotic subtypes’ like H5 or H8, which are unfamiliar to the human immune system, but kept an H1 stalk, which is more familiar.
This means that, once vaccinated, the host immune system is primed to create more broadly protective antibodies that target regions on the HA stalks of the virus.
“The stalk approach is still the one that is the most promising, just because these [stalk] antibodies do exist [naturally in humans],” says Krammer.
In December 2020, Krammer’s first clinical study showed that the vaccine effectively stimulates antibodies against one group of HA in humans and the team is now planning further trials[5].
The stalk approach is still the one that is the most promising
Florian Krammer
Another similar strategy uses just the stalk of the HA protein — an approach being taken by scientists at the US National Institute of Allergy and Infectious Disease Vaccine Research Centre in Bethesda, Maryland.
Their vaccine — H1ssF_3928, based on the H1 protein — displays multiple stalks on the surface of a nanoparticle made from the protein ferritin. Ferritin is useful as a vaccine platform because it forms particles that can display multiple influenza HA spikes on its surface, mimicking the virus itself.
However, there may be problems with these strategies because recent results show that the HA stalk can mutate in some strains.
“We have looked at the potential for mutations in one of the sites in the HA stalk that we are interested in for design of a universal vaccine,” says Ian Wilson, a structural biologist from Scripps Research in California.
“It was clear that, compared to the more antigenic HA head, there’s less mutational ability, but there is some that can lead to escape mutations,” he adds. This occurred with one of most common flu sub-types, H3N2[6].
Creating immunity against multiple flu viruses is complicated by the concept of “original antigenic sin”, or imprinting, Wilson adds.
“Each of us is primed to respond to influenza virus based on what we saw with our first infection as a child … [meaning] that each of us responds differently, depending on our first exposure.
“So, to try to get a completely new response, over a recall response, is more problematic with a virus that circulates in a new form every year.”
Gupta and Craig Thompson, a virologist at the University of Oxford, have developed an approach using a combination of bioinformatics and structural biology to find conserved epitopes in the HA protein head — these are regions of the flu protein recognised by the immune system that have remained constant throughout historic flu strains.
“[Our] model is suggesting [that], rather than there being this continuous arms race, where flu is slowly and incrementally altering the epitopes that it presents to the immune system, it is actually mutating very quickly, but it only has a small number of possibilities within each epitope region,” explains Gupta.
So far, the pair have identified four epitopes that provide immunity to all historical flu strains in mice, while BLU Water vaccines based in Cincinnati, Ohio, is developing a vaccine which would contain a cocktail of those epitopes from a number of flu subtypes[7].
Currently delayed owing to the COVID-19 pandemic (see Box), Gupta is hopeful that phase I trials can start in the near future.
Box: The impact of COVID-19
The COVID-19 pandemic may end up being a game-changer in the push for a universal influenza vaccine, despite the fact that it may have halted flu vaccine progress in the short term, owing to flu laboratories being asked to stop their own work and focus on COVID-19.
According to Wilson, work in flu laboratories has now started to resume and the hope is that the pandemic will provide the push needed to develop better vaccines.
“Vaccines went through two decades of neglect and under-development and we’re feeling the effects of that as pandemics emerge. It has been a dark corner of biotechnology, and it has all of a sudden got a lot of light,” argues Duncan. Now everyone wants to be part of the next Moderna, he jokes.
As the COVID-19 pandemic has progressed, the challenge that viral mutation presents to vaccine development has really come to the fore.
“We initially thought that this coronavirus wouldn’t mutate that much, but we’re now seeing that it is starting to mutate quite extensively, akin to what influenza does on an annual basis,” says Wilson.
Researchers are currently starting to develop prototypes of a universal — or ‘pancoronavirus’ — vaccine, which will work against all coronaviruses, not just SARS-CoV-2. Cambridge, Massachusetts-based VBI Vaccines has announced some promising, if early, results from experiments on animals.
T-cell vaccines and other approaches
Predicting how viral surface proteins will mutate still comes with considerable uncertainties, so researchers are also exploring other avenues.
“There is more to immunity than just stopping infection [with antibodies] … in fact, it may well be that [other mechanisms of] immunity may attenuate the disease process, for example, through cytotoxic T cells,” says McCauley.
Inducing a T-cell response by vaccination has been the aim of recent vaccine research for diseases like HIV and malaria. Bioscience is taking this approach with its vaccine, FLU-v.
T cells are white blood cells that are programmed to attack specific antigens; they also produce signalling molecules, called cytokines, that stimulate a further immune response. This means that vaccines that stimulate a T-cell response can be more effective than those that just stimulate antibody production.
ConserV was able to identify epitopes from proteins inside the virus that activate a T-cell immune response. Its synthetic peptide vaccine is designed to provide broad protection, including against avian and swine flu strains that pose the greatest pandemic risk.
We are antipandemic, by the nature of our design
Kimbell Duncan
“We focus on segments of proteins that do not evolve over succeeding generations of viral strains or as they jump from animals to humans, so we are antipandemic, by the nature of our design,’” explains Kimbell Duncan, chief executive of ConserV.
As well as testing against a broad range of circulating strains, ConserV has successfully completed a phase IIb intranasal challenge study in 153 healthy adults[8].
Trial participants are directly exposed to the 2009 H1N1 flu strain to see the different responses in vaccinated and unvaccinated groups.
“We vaccinated people and then we put them all sort of in a flu hotel,” explains Pleguezuelos.
“This is the first time that a vaccine of this type has shown efficacy [in a human study].”
The trial showed that a single dose of the vaccine led to a larger proportion showing no disease at all compared to those unvaccinated (67.5% versus 45.2%)[8].
Other strategies being investigated include using the surface protein, NA, in addition to HA.
Given the recent success of mRNA vaccines for COVID-19, there is a growing interest in this approach too — a team at the University of Pennsylvania in the United States has developed an mRNA vaccine that encodes for the full HA protein, which was able to induce high levels of stalk antibodies[9].
Future prospects
For ConserV, the current challenge is not identifying the vaccine targets, but defining a regulatory pathway since there is currently no process for approving a flu vaccine with a specific broad spectrum or indeed universal designation.
“Commercially, it’s not viable if we come out of phase III, with the same label that an annual flu vaccine has, we can’t compete,” says Duncan.
Krammer agrees that regulatory issues need attention, but there are inherent difficulties.
“In animals [we] can easily prove that a vaccine is universal, by challenging them with all kinds of subtypes, but how do we prove that in humans?”
A broad-spectrum designation will likely require trials over multiple flu seasons, and “[it takes] a lot of money to do that,” says Krammer.
In animals [we] can easily prove that a vaccine is universal…but how do we prove that in humans?
Florian Krammer
Although a truly universal vaccine is currently a long way off, McCauley sees the potential for a better seasonal one within a “reasonable time frame”.
ConserV is currently in discussions with the US Food and Drug Administration to submit plans for a two-year, phase III trial of Flu-v.
And, Krammer hopes that if the current stalk-focused strategies continue to be successful, a broad-spectrum vaccine should be available within five years.
Even though we can now produce vaccines quicker than ever before, COVID-19 has shown us that millions of people can still die in the time that it takes — and a universal vaccine could prevent this. Bearing recent events in mind, Pleguezuelos argues that now is certainly not the time for complacency.
“This is what we’re trying to do with influenza — we don’t want to wait until there is a pandemic.”
- 11918 Pandemic (H1N1 virus). Centers for Disease Control and Prevention. 2019.https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html (accessed 22 Mar 2021).
- 2Pandemic influenza. World Health Organization. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/pandemic-influenza (accessed 22 Mar 2021).
- 3The Human Vaccines Project Launches New Initiative To Accelerate Development Of Universally Effective Influenza Vaccines. Human Vaccines Project. 2017.https://www.humanvaccinesproject.org/news-item/the-human-vaccines-project-launches-new-initiative-to-accelerate-development-of-universally-effective-influenza-vaccines/ (accessed 22 Mar 2021).
- 4Universal Influenza Vaccine Research. National Institute of Allergy and Infectious Diseases. 2019.https://www.niaid.nih.gov/diseases-conditions/universal-influenza-vaccine-research (accessed 22 Mar 2021).
- 5Nachbagauer R, Feser J, Naficy A, et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med 2020;27:106–14. doi:10.1038/s41591-020-1118-7
- 6Wu NC, Thompson AJ, Lee JM, et al. Different genetic barriers for resistance to HA stem antibodies in influenza H3 and H1 viruses. Science 2020;368:1335–40. doi:10.1126/science.aaz5143
- 7Thompson CP, Lourenço J, Walters AA, et al. A naturally protective epitope of limited variability as an influenza vaccine target. Nat Commun 2018;9. doi:10.1038/s41467-018-06228-8
- 8Pleguezuelos O, James E, Fernandez A, et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. npj Vaccines 2020;5. doi:10.1038/s41541-020-0174-9
- 9Pardi N, Parkhouse K, Kirkpatrick E, et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun 2018;9:3361. doi:10.1038/s41467-018-05482-0