Patients suffering from the chronic inflammatory disorders Crohn’s disease and rheumatoid arthritis have benefited from next generation biologics, such as tumour necrosis factor (TNF) inhibitors, since their development in the late 1990s. However, for around 30% of patients, these once powerful biologics are rendered less effective within a year of beginning treatment and, soon after, the inflammatory disorder flares up again.
Patients are somehow becoming resistant to treatment. One reason is the development of antibodies specific to the active agents in the biologics, which causes increased anti-TNF clearance. Some scientists suspect that protein aggregates found in biologic formulations stored in prefilled syringes might cause this immunogenicity.
“Everything points to aggregation,” says John Carpenter, professor of pharmaceutical sciences and co-director of the Centre for Pharmaceutical Biotechnology at the University of Colorado. “We need to do more monitoring of patients so we can find out what happens to make them suddenly start producing antibodies. Did something happen to the product in one of their syringes and are some patients more susceptible to the effects of aggregation?”
In the cell, in order to function properly, newly synthesized proteins fold into an intricate three-dimensional structure, with a tendency for the hydrophobic parts of the protein to be turned inwards away from the aqueous interior of the cell.
If a protein misfolds, either spontaneously or after experiencing stress, the inner hydrophobic segments can be exposed to the exterior causing misfolded proteins to bunch together to form aggregates.
The challenge for pharmaceutical companies is to come up with a way of controlling the aggregation of proteins within biologic formulations to maintain the functionality of the active agents.
Material problem
Several components within glass prefilled syringes may cause proteins to aggregate and form particles. These include silicone oil, which is used to aid movement of the plunger, tungsten residue, from the metal pins used to form glass in the holes into which needles are glued, needle adhesives and rubber plunger stoppers[1],[2].
“Aggregation can be caused by a number of product and environmentally related factors, and different proteins may be more sensitive to one or another,” explains Satish Singh, a biopharmaceuticals researcher at US pharmaceutical company Pfizer.
With the inexorable rise in the prefilled syringe market — projected to increase from sales of approximately 3.5 billion this year to 12.4 billion in 2024[3] — research to assess the risk of protein aggregation and whether it can lead to treatment failure is important.
A growing understanding of the interactions between therapeutic proteins and silicone oils, air bubbles and other particles inside prefilled syringes is prompting new approaches to formulation and syringe design.
Scientists are using fluid imaging devices, which use an optical system similar to a microscope, to capture detailed pictures of particles that are formed when therapeutic proteins and chemicals used in syringe production interact, which can lead to protein aggregation.
Real response
In early 2014, Carpenter and colleagues reported research aimed at subjecting biologics to the sort of stresses they are likely to sustain during transportation, including air bubbles, agitation and chemicals within prefilled syringes.
They incubated monoclonal antibodies in prefilled siliconised and unsiliconised syringes, with and without air bubbles or glass beads (to add bulk shear forces without the addition of an air bubble). The researchers then used flow microscopy to find particles bigger than 2µm in diameter. Particles between 0.1µm and 10µm may be the most immunogenic and are considered a critical quality attribute.
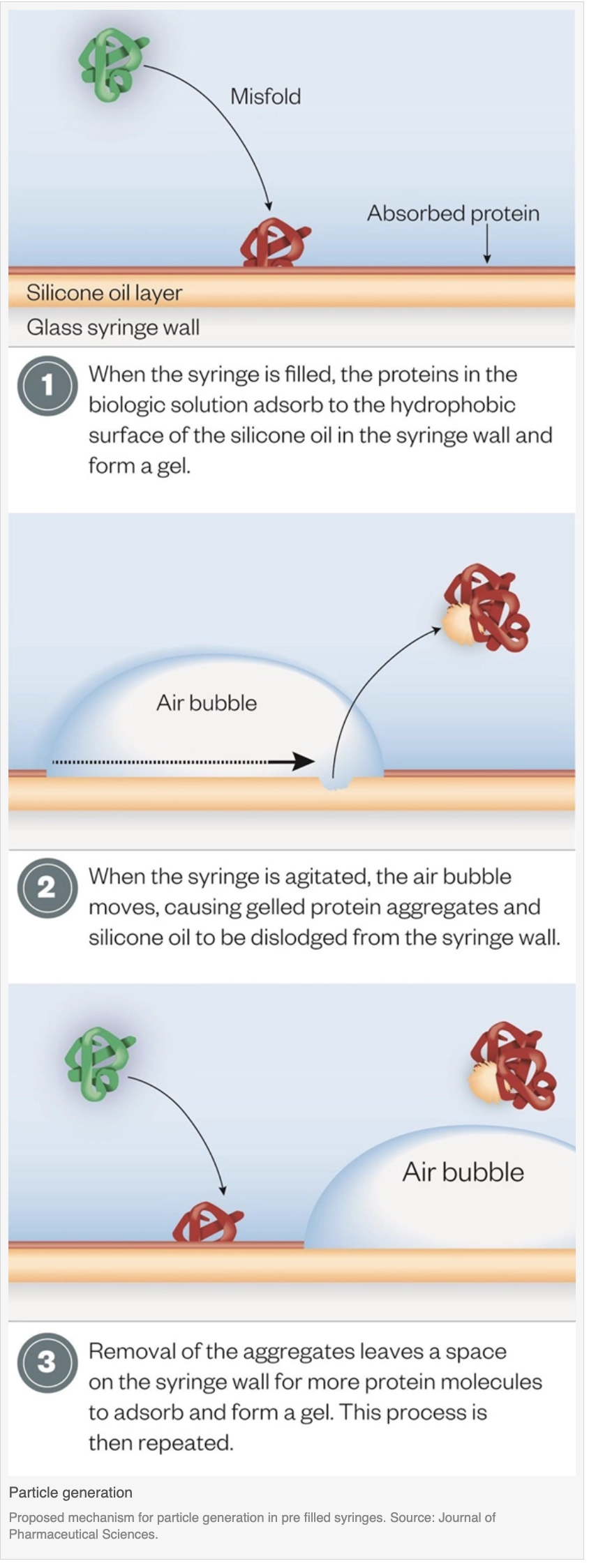
The highest concentrations of particles were found in agitated, siliconised syringes containing an air bubble[4]. Using flow microscopy, the researchers took a closer look at the particles to reveal the clumps were composed of silicone oil droplets and aggregated protein, as well as agglomerates of protein aggregates and silicone oil droplets.
The proteins adsorb to the hydrophobic surfaces of the silicone oil embedded in the siliconised syringes, forming a viscoelastic gel. If an air bubble is added during commercial syringe filling and the syringe is agitated, the air bubble moves along the siliconised syringe wall, disrupting the protein gel as it goes. Gelled protein aggregates and silicone oil droplets dislodge from the syringe wall and into the solution in the syringe, and more protein adheres to the now vacant spot. This process can repeat itself so more and more protein aggregates and silicone oil particles get into the liquid.
Size matters
Data from animal models suggest that immunological consequences depend on the nature of the biologic, the size and amount of aggregates and the state of the protein in the aggregates.
Particles are generally considered visible when they are above 100µm, and US and EU licensing regulations stipulate permissible numbers of sub-visible particles >10µm and >25µm. Among the regulatory agencies, the US Food and Drug Administration (FDA) has begun asking for data on smaller particles in the 2µm to 10µm range.
Until only a few years ago, detecting sub-visible particles was a challenge for pharmaceutical companies because of a lack of suitable techniques. However, advances in measurement technologies have made it possible to look for particles smaller than 0.5µm in diameter during development of biologics.
Singh explains that particles have always been present in parenteral products, and monitoring and controlling for particles over 10µm was introduced because of the risk of blocking capillaries.
“We know that in biologics there are a lot more small-sized particles (<5μm) than larger ones, and we monitor them for quality control and consistency,” he says. “But the impact of these particles on safety and efficacy in humans is difficult to deduce and extrapolate from animal models.”
Patient characteristics also play a role. “A patient on immunosuppressive therapy may not be able to produce a robust immune response, while someone with an autoimmune condition who has an up-regulated immune system may mount a strong response to a mild stimulation,” says Singh. “The patient’s genetic characteristics, such as human leukocyte antigen (HLA) type, can also impact susceptibility, as can concomitant medications.”
Carpenter says the link between particle formation and loss of efficacy remains unproven, and points to the ethical difficulties of deliberately seeking to trigger immunogenicity in patients. However, research by his group has shown a direct correlation between the aggregate or particle content of different interferon-beta products tested in the laboratory and reported rates of neutralising antibody formation in clinical trials[5]. Products with higher rates of antibody formation in clinical trials were found to have the greatest amount of aggregated protein and particles when tested in the laboratory.
By focusing on the structural stability of a protein, it should be possible to make it tougher and more able to withstand any stretching or unfolding at the interface with other molecules, such as silicone oils
Researchers are working to better understand what happens to biologics when they are in solution so that they can design more robust formulations. At the University of Sheffield, chemical engineer Robert Falconer is investigating how interactions between proteins and small molecules, such as those in the excipients used for formulation, affect protein unfolding — the first step in protein aggregation[6].
“If we understand the mechanisms that control protein stability, we can design formulations with the desired properties,” he explains. “By focusing on the structural stability of a protein, it should be possible to make it tougher and more able to withstand any stretching or unfolding at the interface with other molecules, such as silicone oils.”
The big screen
Not all companies developing biologics have the capacity to screen them for aggregates. A way to perform high throughput screening on biologics in development would be of particular interest to small- and medium-sized biopharmaceutical companies that are researching novel biologics but do not have the screening facilities of big pharma.
In the UK, the Centre for Process Innovation (CPI) recently announced a partnership with Arecor, a company specialising in stabilisation of biologic molecules in liquid formulations. The two organisations are going to investigate protein degradation mechanisms and design high throughput screening systems for biologics in development.
“We’re starting with a proof-of-concept study aimed at identifying a simple, practical screening method for early detection of aggregation issues resulting from interaction of proteins with container materials,” explains Guy Casy, director of intellectual assets at Arecor. “We want to perform accelerated stressing on a range of proteins with different grades of silicone oil and under different environmental conditions.”
Jonathan Robinson, head of business development at the National Biologics Manufacturing Centre at the CPI, points to the importance of making decisions early in the development of a new biological candidate to avoid wasted resources later when problems materialise at the manufacturing stage.
Ideal injection
Syringe manufacturers, such as West Pharmaceuticals, headquartered in Lionville, Pennsylvania, and Terumo, headquartered in Somerset, New Jersey, are coming at the problem from a different direction. They are producing plastic prefillable devices that don’t need silicone oil lubricants to facilitate plunger action. Others, such as Becton Dickinson, headquartered in Franklin Lakes, New Jersey, use a cross-linked silicone coating on the inside of their glass syringes that reduces the silicone-related sub-visible particle count in the solution.
However, Singh explains that syringes made of various plastic resins, which eliminate the need for silicone oil, may raise concerns about the potential for chemicals to leach into syringes and the effects of oxygen permeability on the protein solution contained within them.
Nevertheless, some scientists, such as those at Arecor, believe it is just a matter of time before plastic syringes using polymers with properties closer to those of glass become commonplace1.
Whether solving the problem of protein aggregates requires designing more robust proteins and drug formulations, screening out aggregation-prone molecules, modifying syringes or improving patient handling, the ultimate goal is to ensure that biologics remain effective over time.
Handling a problem
Reducing the risk of protein aggregation
Monoclonal antibodies and other therapeutic products are well made and formulated, but they can easily be damaged if, for example, they freeze during transportation, or are exposed to light, high temperatures or vigorous agitation.
“There’s a huge issue about products not being handled properly, and pharmacists can help get the message across to patients that they can’t just collect their medicine and throw it in the back of their car while they go shopping, or leave it on a windowsill when they take it out of the fridge,” says John Carpenter, professor of pharmaceutical sciences and co-director of the Center for Pharmaceutical Biotechnology at the University of Colorado.
As well as following handling instructions themselves, pharmacists can teach patients how to inspect products and ensure they understand how to store and use them correctly.
“Retail and hospital pharmacists can play a very significant role in reducing the potential for aggregation, especially for home-use products,” says Satish Singh, a biopharmaceuticals researcher at Pfizer. “For example, not leaving them under light or in a freezer, or warming them in a microwave or hot water, or shaking them vigorously.”