istockphoto.com
Source: istockphoto.com
Not all the biological targets of a drug are determined when a drug is first developed
In his laboratory at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, Harvard professor Vikas Sukhatme has identified several surface markers found on a type of immune cell called a myeloid-derived suppressor cell (MDSC). These cells cause immunosuppression in cancer patients and can counteract the effects of many of the recently approved immunotherapies to treat cancer. Sukhatme is currently working with a pharmaceutical company to develop an antibody against one of these markers in an attempt to deplete the population of MDSCs.
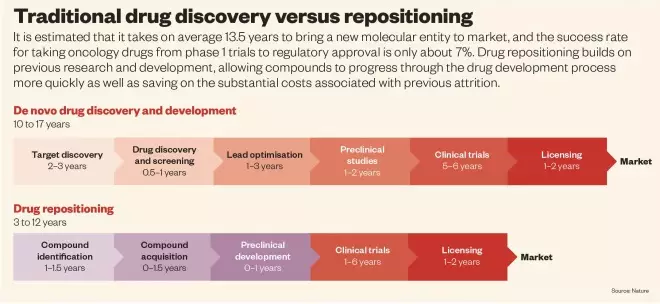
However, developing such a therapy is likely to take many years and success is far from certain. It has been estimated that it takes 13.5 years to bring a new molecular entity to market and, between 2003 and 2011, the success rate for taking oncology drugs from phase I to approval by the US Food and Drug Administration (FDA) was only around 7%.
When an oncology drug does make it to market, companies commonly charge more than US$100,000 for a year’s supply, even though the drugs often provide only modest survival benefits. Oncologists often talk about using combinations of new drugs, but the potentially prohibitive cost of such combinations is much less commonly discussed.
So while Sukhatme continues his research that could lead to novel cancer therapies, he is also considering another option: that some already approved drugs, including generic drugs, that are currently used to treat non-cancer conditions, may also show efficacy in cancer, and do so more quickly, safely and at a much lower cost than new drugs. In many cases, investigators involved in such drug repositioning have been able to jump straight to phase IIa studies, saving at least three to five years, as well as the substantial costs associated with previous attrition, says Aris Persidis, President of Biovista, a drug repositioning company based in Charlottesville, Virginia. This time saving is enabled in the United States by the FDA’s 505(b)(2) regulatory approval pathway, which allows some safety data required for the new indication to come from an approved drug product. Europe has a similar regulatory approval route, the hybrid application based on Article 10 of Directive 2001/83/EC.
Source: Nature
New targets
One reason why a non-cancer drug may prove effective against cancer is that not all of its biological targets may have been identified when the drug was originally developed. The concept of ‘polypharmacology’ has only recently been recognised, says Stephen Naylor, chief executive of MaiHealth, a diagnostics company based in Indianapolis, Indiana. “One [small molecule] drug is likely to have an average of six to seven targets — this is why repurposing is so powerful,” he says. A second reason for the success of non-cancer drugs is the rapidly developing understanding of cancer biology, as scientists discover new targets for cancer therapy.
At the University of New Mexico School of Medicine in Albuquerque, biomedical data scientist Tudor Oprea has been identifying new targets for existing drugs by analysing the similarities in binding sites between different proteins. For example, he noticed that the HIV integrase enzyme and the DNA repair component Metnase have some important similarities in their binding sites. He therefore hypothesised that the HIV integrase inhibitor raltegravir, which has already been approved by the FDA, may also inhibit Metnase. Further analysis, including in silico docking, confirmed this[1]
and led to a pilot study in head and neck cancer in which Metnase inhibition was used to potentiate DNA-damaging chemotherapy[2]
.
One important factor to consider when repositioning drugs is whether efficacy is observed for the new indication at the drug’s previously approved range of dosing levels. “In general, the activity against the newly discovered target is not as high as against the primary, known target,” says Oprea.
Other researchers are also looking for new targets for existing drugs. Sivanesan Dakshanamurthy, an interdisciplinary scientist at Georgetown University Medical Center in Washington, DC, for example, has developed a virtual screening method called RepurposeVS. This platform considers the physical, chemical and thermodynamic properties of both the drug and the target, and can predict which drugs will bind to a selected protein target more accurately than existing in silico docking approaches. “By reducing the false-positive rate, we save time and costs,” he says.
RepurposeVS has identified that mebendazole, an old anti-hookworm medication, is a multi-targeted kinase inhibitor. Mebendazole could be useful if it proves to have a better side effect profile than approved kinase inhibitors that are currently used to treat cancer, or it could be combined with them if it targets additional pathways, says Dakshanamurthy.
Targeting the microenvironment
Until recently, the focus in oncology drug development has been on the tumour cell — first with traditional chemotherapy and then with more ‘targeted’ agents. But much of the focus has now shifted to targeting the tumour’s microenvironment.
“We missed drugs that target the microenvironment because we weren’t looking for them before,” says Pan Pantziarka, a scientist working for the Anticancer Fund, a medical charity based in Belgium. One of the first examples of a successfully repurposed agent in oncology was thalidomide, after it was hypothesised that an anti-angiogenic treatment might show efficacy in the treatment of multiple myeloma by reducing the blood supply to the tumour.
A number of older, approved drugs also target the MDSCs being studied by Sukhatme. These drugs include the PDE5 inhibitor sildenafil (Pfizer’s Viagra) — itself the prototypical example of a repurposed agent — which downregulates the activity of two enzymes that are needed for their inhibitory function. A phase II clinical trial with another PDE5 inhibitor, tadalafil (Lilly’s Cialis), is currently recruiting people with head and neck cancer[3]
.
It would be a shame if just for lack of economic incentive, we don’t give a shot to the dozen or so approved drugs that are also affecting the immune system
Whether these older drugs will be as effective as those being developed specifically to target MDSCs is uncertain. “If you ask most people, they would say the targeted immunotherapy drugs will be more effective,” says Sukhatme. “But it would be a shame if just for lack of economic incentive, we don’t give a shot to the dozen or so approved drugs that are also affecting the immune system. These drugs are relatively inexpensive, and their safety profile, at least for their original indication, is already established. They can immediately be combined with other therapies to assess efficacy, keeping an eye on toxicity.”

Source: Vikas Sukhatme
Vikas Sukhatme and his wife Vidula founded GlobalCures, a not-for-profit organisation that promotes research to find drugs treatments for cancer by repurposing approved medicines
For this reason, Sukhatme and his wife Vidula Sukhatme founded the not-for-profit organisation GlobalCures, based in Newton, Massachusetts, which promotes clinical research to find promising, readily available and cost-effective treatments for cancer by repurposing drugs that are currently overlooked, often because of a lack of financial incentive. In 2014, GlobalCures and the Anticancer Fund together launched a project called Repurposing Drugs in Oncology (ReDO). The aim is to highlight some of the most promising of these drugs, based on in vitro, preclinical and some preliminary clinical evidence in cancer, as well as their plausible mechanisms of action, low toxicity and evidence of efficacy at physiological doses.
So far, ReDO has published papers on the evidence available for a number of drugs and has suggested possibilities for further research. In many cases, the drugs — which include the antifungal itraconazole[4]
, the antibiotic clarithromycin[5]
and the dyspepsia drug cimetidine[6]
— seem to affect aspects of the tumour’s microenvironment. It therefore makes sense to test them in combination with standard-of-care therapies that target the cancer cell. “Maybe we can make a difference by targeting the tumour and the tumour microenvironment at the same time,” says Pantziarka.
Repositioned agents may also be used to target the cancer cell directly — particularly the resistance pathways that limit the effectiveness of targeted therapies in metastatic disease, says Persidis. These pathways may be dysregulated in response to the targeted therapy, or may be intrinsic to the cancer cell. For example, it was recently found that one adaptive mechanism of resistance to BRAF inhibitors in BRAF-mutated melanoma is through the endoplasmic reticulum (ER) stress-autophagy pathway, and targeting components of this pathway can overcome resistance to BRAF inhibitors. The antimalarial drug hydroxychloroquine, which is an inhibitor of autophagy, is currently being tested in combination with a BRAF inhibitor in a phase I trial in patients with melanoma.
Systematic approaches
In order to identify the most important targets in the cancer cell, and to find the best drugs to target them, many researchers are using approaches developed in computational systems biology and systems pharmacology. The ‘brute force’ method of screening large libraries consisting of hundreds of thousands of compounds in vitro to determine which ones cause the death of cancer cells, for example, is costly, often ineffective and unscalable, says Stephen Wong, a systems biologist at the Houston Methodist Research Institute in Texas. “In most cancers, we do combination therapy, and we can’t screen effectively for that,” he says. “A computational approach can guide us towards a much smaller subset of compounds to analyse.”
A computational approach can guide us towards a much smaller subset of compounds to analyse
Additionally, most researchers are seeking more than a single drug–protein interaction. “We are not looking for a single pathway to target, but involving all the relevant pathways and discovering the novel crosstalks between them,” says Wong. Naylor agrees: “Cancer is a system with a whole slew of molecular processes going on, so we need to attack the tumour network, inhibiting several processes at the same time that are vital for the tumour to sustain itself.”
A systems-based and data-driven approach may start with a simple statistical correlation of disease and drug-induced changes in gene expression. “Then it becomes a matching game to identify drugs that may reverse the disease transcriptome or the expression of genes involved in clinically relevant hallmarks of cancer, such as metastasis,” says Craig Webb, chief scientific officer at NuMedii, a company based in Palo Alto, California, that was set up to use this systems-based approach, originally developed by scientists at Stanford University. “This approach is agnostic to our current knowledge of the drug’s target, and we may uncover unexpected but beneficial off-target effects of existing drugs,” he adds.
NuMedii is using this approach to identify drugs to treat the distinct molecular subsets of ovarian cancer. “We have identified some drugs that are predicted to work in specific molecular subsets of epithelial ovarian cancer, which now need to be tested in relevant in vitro or in vivo models,” he says. Wong, meanwhile, has used another ‘knowledge-based’ computational model to identify unique protein signalling networks for three types of breast-cancer metastasis — brain, lung and bone — and has repositioned different drug candidates for each[7]
.
The knowledge-based approach used by NuMedii involves taking a knowledge map of more than 0.5 million highly curated protein–protein interactions, and overlaying the gene-expression profile of the cancer subtype of interest, together with known epigenetic and mutational changes, to identify which proteins are likely to be driving the transcriptional changes observed in the tumour. “Drugs known to target these driver nodes become lead candidates to test further,” says Webb.
Another computational platform, CureHunter, incorporates data on clinical outcomes from more than 1 billion patients taking different drugs to treat different conditions. The data are obtained from clinical trials published in the US National Library of Medicine by using a language processor to ‘read’ all of the library’s 22 million manuscripts. “Unless you connect basic research with clinical outcomes, you are still guessing,” says Naylor, who advises the company. The massive database is an attempt to reveal the connectivity of the entire human disease network across more than 11,000 diseases, he says. It might show, for example, that a drug used to treat rheumatoid arthritis has frequently affected three different protein targets in HER2-positive breast cancer.
The various computational approaches are also being used to discover new drugs, but “repositioning provides an anchor to start with, a known compound with known properties, giving us a head start in the game”, says Naylor. The approaches are too new to have had any success in phase III trials, but it is only a matter of time, says Persidis. “If accidental repositioning has had the significant success it has enjoyed to date, imagine if we apply some science and deploy systematic approaches.”
Accessing compounds
However, in many cases, science may not be the biggest hurdle facing drug repositioning. The first challenge is gaining access to the compounds. One possibility is to access deprioritised compounds that are buried somewhere deep in pharmaceutical companies’ freezers. The National Center for Advancing Translational Sciences (NCATS), part of the US National Institutes of Health (NIH), has developed the New Therapeutic Uses (NTU) initiative, which seeks to make this happen on a wide scale without relying on personal connections between academic researchers and companies. In a successful pilot round in 2012, eight pharmaceutical companies made a total of 58 compounds and their accompanying data available to academic researchers, and NCATS spent US$12m on funding nine projects, mostly phase Ib/IIa trials.
Commercial aspects pose some of the greatest difficulties for drug repositioning because someone else, usually a pharmaceutical company, holds the ‘composition of matter’ patent, giving it the sole right to manufacture the drug for a specific indication or indications. “We typically deprioritise greater than 90% of repositioning candidates for commercial, rather than scientific, reasons,” says Webb.
We typically deprioritise greater than 90% of repositioning candidates for commercial, rather than scientific, reasons
In one common model, the academic investigator or second company will file a new method-of-use patent, negotiate a fee to in-license the compound, conduct early phase II studies, and then give the original company the first right to license the compound back for further development in the new indication.
Financial orphans
There is another, perhaps even greater, challenge if the drug is a low-cost generic or, in Sukhatme’s words, “a financial orphan”. Gaining access to such drugs can be relatively straightforward, but funding costly late-stage, pivotal trials becomes a major issue because drug companies do not anticipate a return on their investment. With no financial incentive to take the drug forward for the new indication, any development initiated by academic investigators often comes to a grinding halt in phase II.
In some cases, achieving the necessary response in the new indication requires a novel formulation of the drug or a new route of delivery, and a new patent may be obtained. Scott Weir, a drug-development specialist at the University of Kansas Medical Center, used his team’s previous industry experience to develop a patented, injectable prodrug of an old topical antifungal agent called ciclopirox, which is selectively delivered to the urinary tract, where it seems to have anticancer activity in bladder cancer. Efforts are now underway to establish a new company to develop the drug. If it is successful, the drug could become the first systemic treatment for non-muscle invasive bladder cancer.
However, in some cases, an old drug found on pharmacy shelves might work perfectly well in a new indication without being modified. This may be the case for auranofin, an off-patent oral rheumatoid-arthritis drug. Weir, in partnership with NCATS and the Leukemia & Lymphoma Society, discovered that auranofin kills chronic lymphocytic leukaemia (CLL) cells. In just 11 months, the partnership, called The Learning Collaborative, took auranofin from laboratory experiments to the clinic. “It definitely would be a great shame if drugs that are very cheap are reformulated and made expensive but are not much more effective,” says Pantziarka.
It definitely would be a great shame if drugs that are very cheap are reformulated and made expensive but are not much more effective
The Illinois-based repurposing-focused charity Cures Within Reach has started connecting funders with researchers in an open, online marketplace called CureAccelerator. The charity’s president, Bruce Bloom, has come up with the idea of a ‘social finance’ initiative — a scheme already in development in the UK — which involves raising money from private investors to fund repurposing trials. The investors are then paid back from the savings in healthcare costs to the government when the repurposed therapies are used.
The government, perhaps working with charities, could fund large drug-repurposing trials where there is no private incentive, especially where there is a chance that the government will make substantial cost savings. In oncology, this may be particularly true for therapies that can reduce the recurrence rate of localised tumours removed by surgery. “It is in the perioperative setting that we can make probably the biggest impact, if we can stop the disease from becoming metastatic,” says Pantziarka.
Perioperative and adjuvant settings
One such example is the non-steroidal anti-inflammatory drug ketorolac, which was found to decrease breast-cancer recurrence from 17% to 6% in a retrospective analysis of 327 women with localised disease when it was given to about half of the patients before surgery to reduce pain[8]
. Ketorolac is being tested in a prospective 200-patient breast-cancer trial in Belgium, supported by the Anticancer Fund, and if a similar risk reduction is observed, the drug could potentially save the lives of 10,000 breast-cancer patients each year in the United States alone, according to GlobalCures. Similar data have also come from a retrospective analysis of non-small-cell lung-cancer patients. One reason why ketorolac might work in the perioperative setting for multiple cancers is that cancer surgery can lead to an inflammatory, wound-healing response, which could potentially result in the metastatic spread of existing cancer cells.
Successful postoperative, or adjuvant, therapy could also save both money and lives by preventing the recurrence of an initially localised tumour. A Cochrane review found that the antacid cimetidine seemed to confer a survival benefit in this setting in colorectal cancer[9]
. But ketorolac and cimetidine are just two of many agents that deserve a proper evaluation in confirmatory studies in the perioperative and adjuvant settings, says Gauthier Bouche, medical director of the Anticancer Fund.
But it is far from clear whether government or large cancer charities will step in to fund such studies, which tend to be large, especially in the adjuvant setting. “Repurposing generic drugs is not typically considered to be exciting or innovative by study sections that peer-review grant applications,” says Weir, who is a member of the NCATS advisory council. “We owe it to patients who lack effective treatments to create a different funding mechanism to identify and advance repurposing opportunities.”
“Generics are an area we need to consider, as it is less likely that the pharmaceutical industry will invest, and it may result in savings for the whole healthcare system,” says Christine Colvis, director of the NTU program at NCATS. However, she adds: “If our sister institutes supported a lot of phase III trials, it would greatly limit the other research they could do.”
One study that has obtained government backing is a randomised, international phase III trial involving more than 3,600 patients that will test whether the old, low-cost diabetes drug metformin can reduce the chance of breast cancer recurrence in those with early-stage breast cancer when given in addition to standard adjuvant therapy[10]
. The US National Cancer Institute is contributing about half of the clinical trial costs of this US$25m trial.
“It became hard to say no [to the trial],” says lead investigator Pamela Goodwin of the NCIC Trials Group in Canada, who worked hard for a decade with colleagues to put together a substantial body of supporting evidence. “The trial would have moved forward ten years earlier if we had a company behind us, but because metformin is a generic drug it took a long time to line everything up to get the study done,” says Goodwin, who also secured funding from charities and philanthropists.
The trial would have moved forward ten years earlier if we had a company behind us
Aspirin is another low-cost, generic drug for which the evidence has built up over many years — particularly with regards to cancer prevention — until it became almost impossible to ignore. The UK National Institute for Health Research, the charity Cancer Research UK and the Medical Research Council’s Clinical Trials Unit have teamed up to spend more than £7m on the 11,000-patient, randomised, controlled Add-Aspirin Trial. In the trial, aspirin is taken after standard adjuvant therapy for early-stage cancers in four different tumour types — breast, colorectal, prostate and gastro-oesophageal — to reduce the rate of recurrence.
If the trials of generic drugs in new indications are successful, one remaining question is whether to go through the costly regulatory process of amending the drugs’ labels, something that is usually done by companies. Many expect that a positive outcome will lead to the revision of major guidelines, such as those of the National Comprehensive Cancer Network, which could lead to a change in clinical practice with the drugs being prescribed or used off-label. Pantziarka, however, is concerned that “even good phase III results may go nowhere” without formal regulatory approval.
In the UK, the Off-patent Drugs Bill would have required the secretary of state for health to secure licences for off-patent drugs in new indications, followed by an appraisal by the National Institute for Health and Care Excellence, but the bill stalled in parliament. Pantziarka hopes that it will be brought forward again: “The campaign for the bill continues, and there is every reason to believe that it will go to parliament again.”
References
[1] Williamson EA, Damiani L, Leitao A et al. Targeting the transposase domain of the DNA repair component metnase to enhance chemotherapy. Cancer Research 2012;72:6200–6208.
[2] University of New Mexico Cancer Center. Pilot study of raltegravir and cisplatin in squamous cell carcinoma of head and neck. ClinicalTrials.gov identifier NCT01275183.
[3] National Institute of Dental and Craniofacial Research (NIDCR). A phase II trial of tadalafil in patients with squamous cell carcinoma of the upper aero digestive tract. ClinicalTrials.gov identifier NCT01697800.
[4] Pantziarka P, Sukhatme V, Bouche G et al. Repurposing Drugs in Oncology (ReDO) — itraconazole as an anti-cancer agent. e cancer 2015;9:521.
[5] Van Nuffel AMT, Sukhatme V, Pantziarka P et al. Repurposing Drugs in Oncology (ReDO) — clarithromycin as an anti-cancer agent. ecancer 2015;9:513.
[6] Pantziarka P, Bouche G, Meheus L et al. Repurposing drugs in oncology (ReDO) — cimetidine as an anti-cancer agent. ecancer 2014;8:485.
[7] Zhao H, Jin G, Cui K et al. Novel modeling of cancer cell signaling pathways enables systematic drug repositioning for distinct breast cancer metastases. Cancer Research 2013;73:6149–6163.
[8] Forget P, Vandenhende J, Berliere M et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesthesia & Analgesia 2010;110:1630–1635.
[9] Deva S & Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database of Systematic Reviews 2012;8:CD007814.
[10] National Cancer Institute. A phase III randomized trial of metformin vs placebo in early stage breast cancer. ClinicalTrials.gov identifier NCT01101438.


