Brain light / Alamy Stock Photo
First, Anna Andreou feels it coming: “I get speech problems, I find it difficult to say phrases”. That’s the premonitory phase. After this, the pain hits. “[It’s] really disabling. You just want to enclose yourself in a dark room.” Andreou has suffered from migraines since she was a child, which is one reason why she decided to specialise in studying the condition – she is currently based at King’s College London.
To treat her migraine attacks, Andreou has limited options. Triptans, the main class of anti-migraine drugs, do not work on her. “I might try some over-the-counter analgesics,” she says. Even if triptans did work, they can have some nasty side effects. And there is nothing currently on the market specifically developed to prevent migraines from occurring in the first place.

Source: Courtesy of Anna Andreou
Anna Andreou, a researcher at King’s College London, says uncovering the pathophysiology of migraines is vital
This may be about to change. Several drugs that target calcitonin-gene-related peptide (CGRP), a small protein that acts as a neurotransmitter, have shown promise in phase II trials and are currently in phase III trials. There is hope that they could enter the clinic within the next couple of years.
Migraine is an intense, throbbing headache that often affects one side of the head and may be accompanied by neurological symptoms such as disturbed vision or, more rarely, speech problems like the ones Andreou suffers from. These neurological symptoms characterise migraine with aura, a subset of the condition. Around 8 million people in the UK have disabling migraines and a further 2–3 million have very disabling migraines. “That’s quite a lot — it’s more than epilepsy, asthma and diabetes together,” says Fayyaz Ahmed from the charity The Migraine Trust, based in London.

Source: Courtesy of Fayyaz Ahmed
Fayyaz Ahmed from the charity The Migraine Trust, London, says more people in the UK suffer from migraines than from epilepsy, asthma and diabetes together
Triptans were developed in the 1980s. They selectively activate 5-hydroxytryptamine (also known as serotonin) 1B/1D (5-HT1B/1D) receptors. They are effective in many (around 70%, although changing formulation may increase that percentage), but not all, migraine sufferers and side effects such as drowsiness, dry mouth, chest tightness and flushing can be a problem. Since they constrict blood vessels, triptans are contraindicated in anyone with cardiovascular disease.
Once patients get over about one migraine a week, they start getting tired of the whole thing and start thinking about prevention
And for around a third of patients, says Peter Goadsby, a neurologist at King’s College London, acute treatment is simply not good enough. “Once [patients] get over about one [migraine] a week, they start getting tired of the whole thing and start thinking about prevention,” he says. Some medicines can be repurposed as migraine preventatives – the most commonly prescribed are beta blockers, which are roughly 70% effective at reducing migraine frequency by more than 50%. Others include anti-epilepsy drugs and antidepressants. These preventatives “have their utility but came from somewhere else and bring a lot of baggage”, says Goadsby. Botox (botulinum toxin) is a proven treatment to prevent severe, chronic migraine, but it requires painful injections every 12 weeks, not to mention the fact that it is the deadliest toxin known to man. “So pick your drug and pick your poison, basically, because all of them have a lot of problems,” adds Goadsby.
A new target
Goadsby has been involved in CGRP blockers from the beginning through his work with Lars Edvinsson, a neurologist at Lund University Hospital in Sweden, who was the first to suggest that CGRP may have a role in migraine. In 1990, Edvinsson teamed up with Goadsby to test his theory. “We met at a meeting and thought that either CGRP or substance P would be important [in migraine],” says Goadsby.

Source: Courtesy of Lars Edvinsson
Lars Edvinsson, a neurologist at Lund University Hospital in Sweden, was the first to suggest that CGRP may have a role in migraine
The two scientists measured the levels of various neuropeptides, including both substance P and CGRP, in blood taken from the jugular vein in migraine sufferers while they were having an attack[1]
. They found high levels of CGRP; nothing else they measured was significantly changed. “Everyone was saying it’s substance P, I was like a lonely soldier at the conferences for about ten years,” says Edvinsson.
The first anti-CGRP drug developed was a small-molecule inhibitor called olcegepant (or BIBN 4096 BS). In a double-blind randomised trial published in 2004, researchers, including Goadsby, reported that a group of migraine sufferers given 2.5mg of the drug for acute treatment had a 66% response rate, compared with 27% for placebo[2]
. There were no serious adverse events.
However, olcegepant, developed by Boehringer Ingelheim (based in Ingelheim am Rhein in Germany), was later discontinued because of trouble developing an oral formulation. Another early CGRP inhibitor, telcegepant, developed by Merck (headquartered in Kenilworth, New Jersey), also ran into difficulties. Edvinsson recalls how Merck were getting ready to submit its files for approval and the US Food and Drug Administration (FDA) asked it to conduct a further trial giving patients two doses every day for three months. “After a month, some patients had an increase in their liver enzymes,” he says. That stopped development in its tracks, although the liver toxicity was probably an off-target effect of the specific formulation rather than a problem with the whole class of drugs, according to both Goadsby and Edvinsson.
Pharmaceutical companies changed tack, developing a series of monoclonal antibodies to bind and neutralise either CGRP itself or its receptor. Four of these monoclonal antibodies are now neck and neck in phase III trials — AMG 334 (co-developed by Novartis, based in Basel, Switzerland, and Amgen, based in Thousand Oaks, California), TEV-48125 (Teva, based in Petah Tikva, Israel), ALD403 (Alder Biopharmaceuticals, based in Bothell, Washington), and LY2951742 (Eli Lilly, based in Indianapolis, Indiana) (see table 1). Edvinsson describes the situation as similar to “four race horses and they are about the same level”.
| Name | Company | Target | Main phase II findings | Expected phase III results |
|---|---|---|---|---|
| AMG-334 | Novartis/Amgen | CGRP receptor | Mean change from baseline in migraine days in weeks 9–12 of a 12-week study was -6.6 days in both high and low dosage groups compared with -4.2 days in the placebo group. | No longer recruiting, estimated completion date March 2017 |
| ALD403 | Alder Biopharmaceuticals | CGRP | Mean change from baseline in migraine days between baseline and weeks 5–8 of an 8-week study was -5.6 days in the drug group compared with -4.6 days for the placebo group | Currently recruiting, estimated completion date June 2017 |
| LY2951742 | Eli Lilly | CGRP | Mean change from baseline in migraine days between baseline and weeks 9–12 of a 12-week study was -4.2 in the drug group compared with -3.0 in the placebo group | Currently recruiting, estimated completion date June 2017 |
| TEV-48125 | Teva | CGRP | Mean change from baseline in headache hours in weeks 9–12 of a 12-week study was -67.51 hours in the high dose group compared with -37.10 in the placebo group | Currently recruiting, estimated completion date October 2018 |
There’s a crystal clear advantage — they’re much better tolerated than triptans
All the anti-CGRP drugs in development seem to show efficacy. For example, ALD403, a monoclonal antibody to CGRP itself, led to a 75% reduction in migraine days over the course of a 12-week phase II trial in around 30% of patients. The drugs are delivered via either subcutaneous or intravenous injection; just one intravenous injection of ALD403 delivered in the clinic can protect patients from migraine for six months. And human studies show that CGRP receptor antagonists are not vasoconstrictors. Compared with triptans, says Goadsby, “there’s a crystal clear advantage — they’re much better tolerated”, although he acknowledges that it is too soon to know if there could be any long-term adverse effects.
The clue for Edvinsson that CGRP might be involved in migraine came from the fact that it is highly prevalent in the trigeminal system, the sensory nerves that supply the head and neck. But he was following a hunch: the pathology of migraine remains deeply mysterious. Andreou remembers getting excited during her undergraduate studies about a lecture on migraine, thinking she might learn the mechanisms underlying her condition. She was quickly disappointed: “The first thing the lecturer said is we still don’t know what causes it.”
Competing theories
If you have ever experienced a migraine, the pain is pulsating — it’s hammering — and it goes with the tempo of your pulse
In the second half of the 20th century, thinking about migraine was dominated by what is known as ‘the vascular theory’, which suggested that the excruciating pain that defines the condition is caused by dilation of vessels around the head or in the brain, or both. This makes intuitive sense: “If you have ever experienced a migraine, the pain is pulsating — it’s hammering — and it goes with the tempo of your pulse,” says Aarno Palotie, a geneticist at the Center for Human Genome Research at the Massachusetts General Hospital in Boston.
However, not all the evidence supports the vascular theory. In 2013, a Danish team that included Faisal Mohammad Amin, a neurologist based at the Danish Headache Centre in Copenhagen, reported on a study in which MRI scans were performed on people undergoing spontaneous migraine attacks[3]
. “We found no change of the extracranial arteries,” says Amin.
The alternative to the vascular theory, the neuronal theory, suggests that the root of the problem lies in the brain. It was first proposed in the late 19th century but was largely displaced by the vascular theory, regaining popularity in recent years. Andreou believes that the hypothalamus and thalamus are two brain regions that will turn out to be vital in understanding migraine.
If you change the sleep patterns you have, or the food patterns you have, that can be a trigger for migraine
Migraine sufferers often report that certain things can set off an attack. “If you change the sleep patterns you have, or the food patterns you have, that can be a trigger for migraine,” says Andreou. She believes that these triggers represent deviations from the body’s natural balance, or homeostasis, “which is controlled by the hypothalamic area”. This could be one reason why migraine is about three times more common in women than men after puberty (the rates are about the same before). “A lot of women will get their migraine attacks near their periods,” Andreou notes, when there are hormonal changes. “Again it’s deviation from homeostasis.”
The hypothalamus can influence the thalamus, a deep brain structure that can control a number of different functions including pain, but also sensitivity to light and noise, both of which can be affected in migraine. “If you think about their light sensitivity,” says Goadsby of a hypothetical migraine patient, “you conclude they don’t get more light than you or I, not a single photon extra.” Because the thalamus can act as a ‘gateway’ to sensory stimuli, he too believes that it could be involved in migraines.
Something everyone can agree on is that genetics are important. “Migraine patients tend to have a first degree relative who’s got migraine,” says Goadsby. Palotie has recently led a large study into the genetics of migraine, which identified 38 distinct genomic loci associated with migraine[4]
. Many of those related to genes expressed in vascular or smooth muscle tissue. “We do find a strong association to genes and gene areas which are linked to vascular function,” says Palotie. However, he cautions against assuming that there is no link with neuronal function. “If you don’t see something it doesn’t mean that it doesn’t exist,” he says. Ultimately, perhaps both theories will turn out to be partly right. Palotie believes this may well be the case, and for Goadsby: “It doesn’t matter who’s wrong, it only matters that you get it right.”

Source: Courtesy of Aarno Palotie
Aarno Palotie, a geneticist at the Center for Human Genome Research at the Massachusetts General Hospital in Boston, has recently led a large study into the genetics of migraine
So where does all this fit in with the CGRP story? The answer is that there is no obvious answer. CGRP is found throughout the body and the brain. Although particularly prevalent in the trigeminal system, there are also abundant CGRP receptors in the cerebellum – and its physiological role is unclear.
It is not even clear where anti-CGRP medications have their effect. Gepants are small enough to partially cross the blood–brain barrier, but the monoclonal antibodies are too large, at least under physiological conditions. This would suggest that they work by removing CGRP from the periphery. Edvinsson believes that their probable site of action in migraine relief is the trigeminal ganglion, which he compares to “a tuner of the radio”, pointing out that even if your radio is not tuned in, the signals are still there. Therefore, the brain could still be having a migraine attack but the pain of that attack is blocked.
Goadsby, however, believes that there could still be some central effects. “It’s pretty clear that the blood–brain barrier is altered in migraine,” he says. “I don’t think it’s likely that there’s a single site of action.”
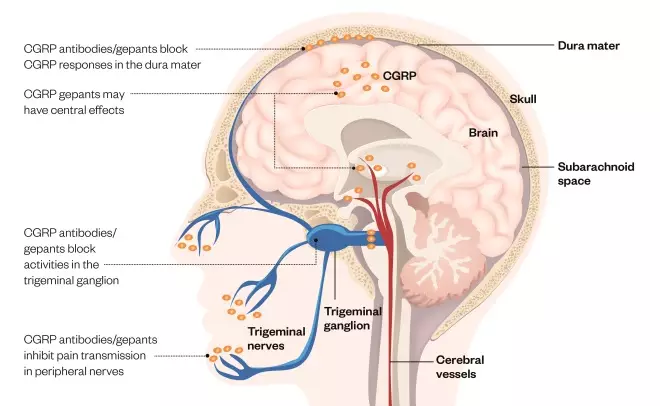
Figure 1: Targeting CGRP in migraine
Source: The Pharmaceutical Journal
The neurotransmitter calcitonin-gene-related peptide (CGRP) is present throughout the body and brain, and spikes during migraines. CGRP antibodies and antagonists act by reducing CGRP levels or blocking the actions of CGRP, although it is not clear where they have their effect. CGRP antibodies are thought to be peripherally restricted, whereas CGRP antagonists — the gepants — may have central actions
Possible complications
Not everyone is as positive as Goadsby and Edvinsson about the possibilities of blocking CGRP. “I’m a bit disappointed by the outcomes of the phase II trials,” says Andreou. “I was expecting to see better efficacy.” She believes there probably will be a role for anti-CGRP agents in the clinic but “it’s not the golden pill – or golden injection in this case”. In her opinion, botox — a treatment reserved for those with very severe, chronic migraine because of its expense and toxicity profile — will remain the best treatment for migraine. Andreou explains that both CGRP and glutamate are involved in activation of the pain pathway and “with botox we’re blocking neurotransmitter release completely”. She is currently working on a new botox molecule that has no paralytic actions.
Another concern is that, with long-term exposure to the anti-CGRP antibodies, patients could develop antibodies to the antibody treatments themselves. Goadsby acknowledges that “there will certainly be some patients who develop antibodies” but says that the extent of the problem, and its potential clinical significance, remain unknown because phase III trials are ongoing.
Goadsby is optimistic about the timeline for the first anti-CGRP drug reaching the clinic: “My guess is that it will be somewhere in the second half of 2018.” He believes the FDA understands the importance of these potential new medicines and is primed to approve them as quickly as possible once all the phase III data are in.
Because migraine doesn’t kill you, healthcare professionals don’t seem to be too serious about treating them
For patients, “the main frustration is being told it’s just a headache”, says Ahmed from The Migraine Trust. “Because migraine doesn’t kill you, healthcare professionals don’t seem to be too serious about treating them.” CGRP antagonists, like other migraine treatments, are not going to be a cure but Goadsby sees them as “a huge step forward”. “It’s going to mean migraine treatments for migraine patients, and that’s what they don’t have,” he says.
For Andreou, who, all those years after her first lecture on migraines, is still trying to understand her own condition, uncovering the pathophysiology of migraines is vital. She says: “A lot of patients come to the clinic and ask what is causing it. We owe a better answer to those patients.”
References
[1] Goadsby PJ, Edvinsson L & Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–187. doi: 10.1002/ana.410280213
[2] Oleson J, Diener HC, Husstedt IW et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004;350:1104–1110. doi: 10.1056/NEJMoa030505
[3] Amin FM, Asghar MS, Hougaard A et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol 2013;12:454–461. doi: 10.1016/S1474-4422(13)70067-X
[4] Gormley P, Anttila V, Winsvold BS et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 2016;48:856–866. doi: 10.1038/ng.3598


