Mclean
Sandy Douglas, one of a handful of scientists at the heart of the University of Oxford’s efforts to produce a vaccine against COVID-19, was working on rabies when SARS-CoV-2 began to stir in December 2019.
An academic clinician, Douglas had hired a postdoctoral researcher five months earlier to improve the yield of their adenovirus-vectored rabies vaccine. The volumes they were working with were tiny — just 30mL — but Douglas knew that the manufacturing process was scalable in theory. By early January 2020, they’d had some success in the laboratory, increasing vaccine yield substantially by tweaking the medium used. “At that stage, I wasn’t thinking about coronavirus,” he says.
Elsewhere in the university, the emerging pathogens vaccine research group, led by Sarah Gilbert, had started to design a vaccine against SARS-CoV-2 using the ChAdOx1 viral vector. Scientists in China published the genetic structure of the new coronavirus on 10 January 2020 and, within weeks, Gilbert’s group had created a vaccine — ChAdOx1 nCoV-19 — that induced an immune response in the lab.
Even if there was a 10% chance that the numbers from China were right, the outbreak of SARS-CoV-2 in Wuhan was very bad news
Sandy Douglas, academic clinician, University of Oxford
Around the same time, convinced that a pandemic was looming, Douglas changed his focus. “I became very anxious,” he says. “Even if there was a 10% chance that the numbers from China were right, [the outbreak of SARS-CoV-2 in Wuhan] was very bad news.”

University of Oxford/John Cairns
Douglas started talking with Gilbert about how the university could accelerate small-scale manufacturing for clinical trials and hatched a plan to have a phase I batch of ChAdOx1 nCoV-19 ready for March/April 2020.
For Douglas and Gilbert, there was near certainty that the technology would produce an effective vaccine. “We can just drop in another gene and get an antibody response,” says Douglas. “We’ve done it for literally hundreds of things.”
What they hadn’t done was manufacture at scale. “I don’t think anyone else would have viewed us as being credible to scale up,” says Douglas. “Our only publication on a scalable adenovirus vector manufacturing process was [by] me [in] 2019, in a fairly obscure journal.”
Douglas and his colleagues ploughed ahead, presenting their initial vaccine manufacturing plan to the weekly departmental meeting.
We put up a slide that was basically . . . the idea that we could go from vaccinating one person to 1,000 people within a month
Sandy Douglas, academic clinician, University of Oxford
“All the profs who are anything to do with infectious disease would come and sit around the table and talk about what we were going to do about this [global health emergency]. I was by far the most junior person in the ring, the only non-prof,” recalls Douglas. “We put up a slide that was basically bringing together this accelerated clinical trial manufacturing, and the idea that we could go from vaccinating one person to 1,000 people within a month.”
At the time, vaccines against COVID-19 were considered a long-term goal, unlikely to be available for another 18 months. But Douglas and his colleagues were confident that they could expand the trials rapidly with a high degree of safety. “In February 2020, that was a pretty revolutionary idea,” he says.
Development scale up
As the strategy for accelerating trial manufacturing fell into place, Douglas’s focus shifted again. With his colleague Cath Green, who runs the university’s clinical biomanufacturing facility, he sought help from the UK Bioindustry Association. Green, who has contacts within the association, sent an email on Douglas’s behalf, appealing for help with the development scale up and manufacturing of a potential vaccine.
Alongside this appeal, Douglas put in a grant application to UK Research and Innovation for £400,000 to support their scale up plans. “We had a result at the 30mL scale and we were proposing to go to 200L [using Good Manufacturing Practice standards], to make a million doses before the end of 2020,” says Douglas.
As the grant application was processed, offers of help started to land. Pall Biotech, a biotechnology company based in Portsmouth, would develop the university’s ChAdOx1 nCoV-19 vaccine manufacturing process, scaling to 50L and then to 200L. Also on board were contract manufacturers Cobra Biologics and Oxford Biomedica in the UK, and Halix BV in the Netherlands. Working in parallel with Pall, the technology would be transferred to Cobra, Oxford Biomedica and Halix, who would then manufacture the vaccine candidate using Good Manufacturing Practice standards.
“We set up this pretty unusual, unfunded consortium,” explains Douglas. “We had a sort of SharePoint or OneDrive system with a multilateral confidentiality agreement, which basically said everyone can share everyone’s information and work in a very, very cooperative way, which was essential.”
Within that first conversation, it was clear to me that Sandy’s team had done something that was different from a lot of academics
Daniel Smith, chief scientific officer at Cobra Biologics
Daniel Smith, chief scientific officer at Cobra Biologics, remembers the first time he spoke to Douglas: “Within that first conversation, it was clear to me that Sandy’s team had done something that was different from a lot of academics — they’d got a scalable solution in place, albeit at a very small scale and unproven.”
Plans were immediately set in place, with discussions focused around what needed to happen, when and who would pay. “[Businesses] came on board at significant financial risk,” says Douglas. “It was very generous and trusting of people.”
The consortium’s aim was to scale up to 200L, then set up parallel manufacturing runs. “If we ran multiple streams at multiple sites, we’d get a nice amount of material made in those timeframes,” explains Smith. However, while 200L wasn’t massive in terms of bioproduction, it was large for viral production. Cobra did not have the facilities in-house to go to 200L, so Smith drew on personal connections to drive the plan forward. “I rang the director of strategy [at Pall] who I know very well, a guy called Clive Glover.” Smith asked Glover if he would lend him a bioreactor to enable the scale up to 200L. “He said: ‘Yes, we can put one on a cart tomorrow and ship it to you.’ It was literally as quick as that.”

Philip Gatward
Things moved rapidly from that point, recalls Smith. “Sandy was trying to co-ordinate this massive effort across multiple sites to do process development in his lab, current parallel scale up at Pall Life Sciences, tech transfer into Cobra and the other [contract manufacturing organisations], all of the equipment procurement, all of the raw materials procurement, and the strategy for how we could move very quickly in a regulated environment.” (See Box).
Box: Delivering efficiencies and tackling bottlenecks
Close working between the different domains of drug development — preclinical, clinical and manufacturing — allowed the team at the University of Oxford to identify a critical path to large-scale vaccine production. By separating out the management of business and financial risks from patient safety, they were able to shave months off the manufacturing process, says academic clinician Sandy Douglas.
For example, whenever a material, such as cells or the virus, moves from one facility to another, thorough testing is done to demonstrate that there are no contaminants. “That testing is about business risk, rather than safety risk; you can move the thing to the new facility, get it started and do the testing in parallel,” Douglas explains. “If the testing result comes out bad, you’ve lost a load of money and your facilities need deep cleaning, but you’re going to have the result before it goes anywhere near a patient. We saved months through doing that again and again and again.”
Single-use bioreactors, which make use of plastic bag technology, have also delivered efficiencies. They remove the need to deep clean plants between production runs and have facilitated parallel batch manufacturing. As demand for a product increases, instead of increasing batch sizes and having to verify that the increase did not affect the manufacturing process, multiple batches are run in parallel.
Collaboration across academia, industry and government has also moved things forward at speed. The UK government’s Vaccine Taskforce recognised early on that fill finish — the transfer of drug product into vials — was a potential bottleneck in the manufacturing process and that vaccine manufacturers were unlikely to have the necessary capacity or capability. To this end, the government partnered with global pharmaceutical and biotechnology company Wockhardt in August 2020, to provide fill and finish services from its facility in North Wales.

Wockhardt
Speaking at a Science Media Centre briefing on vaccine manufacturing on 11 March 2021, Ian Muir, a contract manufacture specialist for the Vaccine Taskforce, explained: “We set out early to make sure that we had a robust supply chain, to not only take the bulk vaccine and put it into vials, but also so we had enough vials and stoppers to make sure that we could manufacture enough at scale in the UK.” Muir, who is chief executive of Porton Biopharma, added: “That was something that was done very early on in the programme, before we even knew whether there’d be a vaccine or what the vaccine might look like.”
Daniel Smith, chief scientific officer at Cobra Biologics, recalls a significant milestone for the Oxford vaccine consortium that illustrates the impact of these efficiencies: “From that initial conversation with Sandy on 25 February 2020, to when Cobra made the first 200L Good Manufacturing Practice batch and released it out of our facility, was 109 days. That’s phenomenal.”
Competitors were working as a harmonious team, pulling towards a common objective, says Douglas, but still with no money. “We were having calls almost every day, [and] a weekly steering group… with the senior people from each organisation,” he says. “There was an overwhelming business case that this was going to make sense. But it was running on empty.”
Despite this, and the fact that no one was really sure 200L could be achieved in the timeframe proposed, Douglas was already thinking about how the consortium could go beyond 1 million doses.
I’ve got an Excel spreadsheet that’s called ‘billion dose scale’ from 2 March 2020. But I didn’t think anyone would take it seriously
Sandy Douglas, academic clinician, University of Oxford
“I’ve got an Excel [spread]sheet that’s called ‘billion dose scale’ from 2 March 2020. It’s a back-of-an-envelope calculation,” he says. “But I almost didn’t talk about it. I didn’t think anyone would take it seriously.”
The limiting factor was credibility, says Douglas, and what they could persuade people to believe an academic-led consortium might be able to do. Later that month, money began to trickle in as the UK Research and Innovation grant was awarded.
Tipping point
In April 2020, the UK government set up the Vaccines Taskforce (VTF). It had three main objectives: to secure access to the most promising vaccines for the UK population as quickly as possible; to make provision for international distribution of vaccines; and to establish a long-term vaccine strategy to prepare the UK for future pandemics.
Douglas met with the VTF’s manufacturing subgroup, presenting the consortium’s projections for supplying the UK with vaccines and setting out the £65m costs involved. “A few days later, I got a one-line email from a civil servant saying you can have 65 million quid,” he says.
Douglas describes it as a tipping point. Governments were realising that they needed to buy vaccines before anyone knew whether or not they would work. And they needed to pay for the manufacturing scale up. “We’d gone from having nothing, to having … an enormous amount of money,” says Douglas.
We were able to negotiate a deal that said, during the pandemic, it’s going to be at cost price
Sandy Douglas, academic clinician, University of Oxford
With government funding to support its UK ambitions, the university was able to push its global agenda. To achieve their goal of creating a vaccine to help the world, the team at Oxford would need billions of doses and a heavyweight industry partner. “With government money, the university was in a very strong position in the negotiations with AstraZeneca,” says Douglas. “We were able to negotiate a deal that said, during the pandemic, it’s going to be at cost price.”
As the Oxford vaccine entered phase I trials in April 2020, the university signed a deal with AstraZeneca. Under the agreement, the Anglo-Swedish pharmaceutical giant would be responsible for development, worldwide manufacturing and distribution of the vaccine.
Smith remembers the AstraZeneca teams joining their tech transfer meetings. “They do, and they did, feel just like another partner coming in at that point; they wanted to listen, they wanted to understand what we were doing,” he says. “They’re a bunch of scientists; everyone wants to talk about how to make it better.”
Any fears that a large pharmaceutical company would slow things down were not borne out. “Everything has to go through a lot of control points,” explains Smith. “They still do that, but they keep it away from us. And they move phenomenally fast.”
Douglas describes the relationship with AstraZeneca as very positive. “They have brought in a lot of technical capacity, expertise and experience that we didn’t have,” he says — including the capacity to transfer the required technology to dozens of contract manufacturers.
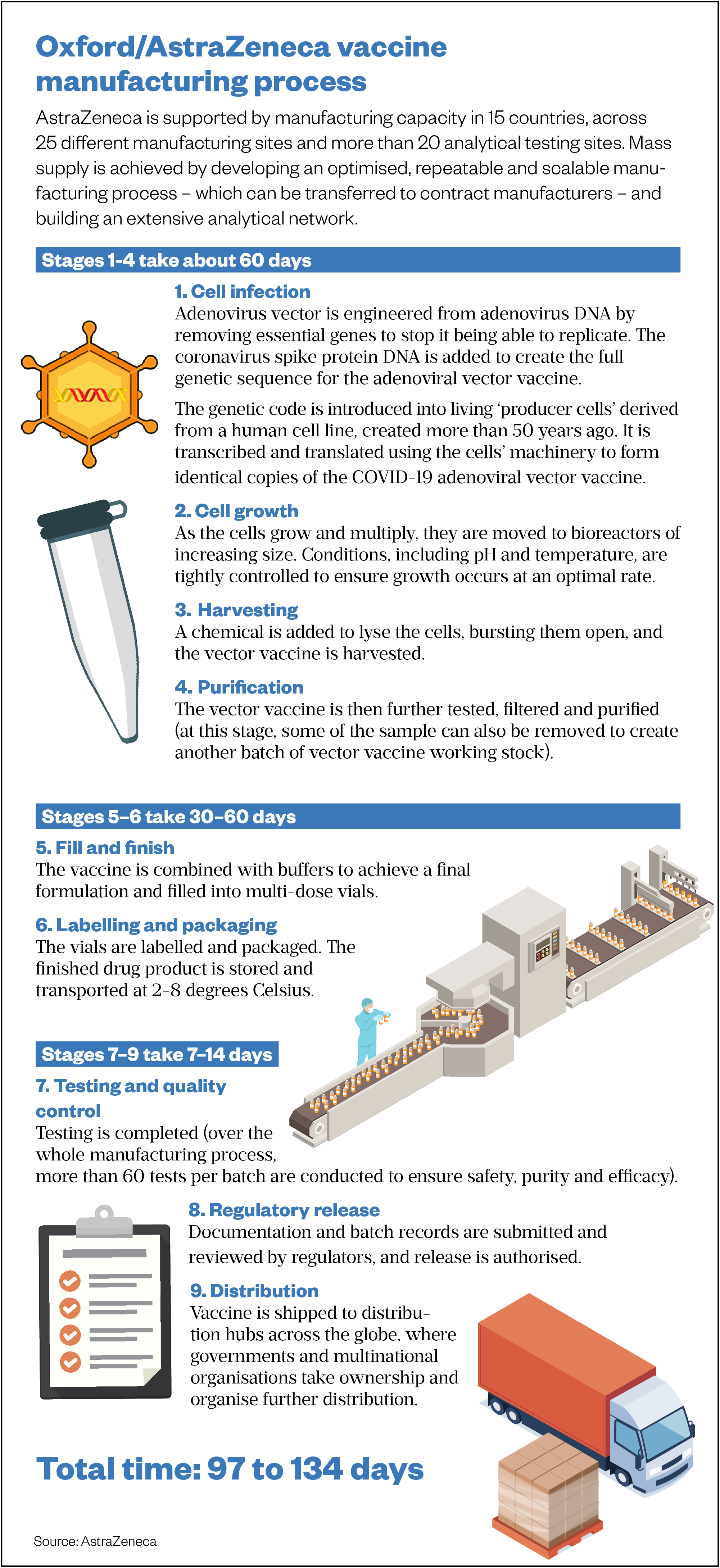
The aim from the beginning was that Cobra, Halix and then Oxford Biomedica would be doing mass supply, says Douglas. “That plan has been taken over wholesale by AstraZeneca. It’s been revised and updated, obviously, and made more ambitious, but the fundamental plan to supply the UK was [already in place].”
AstraZeneca has continued to work with the consortium’s manufacturing partners, using a model of distributed production (see Figure). “They extended that model by more or less contracting every manufacturing site that had the capacity to work with this virus globally and transferring to them the same process that we’d developed for the consortium in the UK,” explains Douglas.
With around two dozen manufacturing partners, including the world’s biggest vaccine manufacturer the Serum Institute of India, AstraZeneca plans to produce up to three billion doses of the vaccine in 2021. The agreement with the Serum Institute of India, alongside the COVAX programme — which is being co-led by the Coalition for Epidemic Preparedness Innovations, Gavi, the Vaccine Alliance and the World Health Organization — will support equitable global access to the vaccine for low- and middle-income countries.
Source: AstraZeneca
Smith hopes the UK will learn lessons from the pandemic and put processes in place to make academic translation of medicines far smoother and quicker. “The value [of medicines] comes not just from the medicine itself, but the ability to make that medicine and distribute that medicine. We need to … capture all of that.”
From the start of the pandemic, the University of Oxford’s ambition was to make a vaccine for the world and — despite a few bumps in the road — a little over a year later, it has gone a long way to achieving that.