
K H Kjeldsen / Science Photo Library
After being bitten by ticks in her Devonshire garden, a spreading rash appeared on Stella Huyshe-Shires’s hip. Soon afterwards, her health began to deteriorate but she had no idea what was causing her pain, lack of energy and other problems. It was three years before a positive blood test confirmed Lyme disease, a test Huyshe-Shires asked for after recognising her symptoms in a letter about the spread of the tick-borne illness in a local newspaper.
“I was getting lots of things wrong with me and it’s not the sort of thing you expect to happen to you until you’re 80,” explains Huyshe-Shires, who was 48 years old when she got the rash. By the time she was diagnosed, her list of ailments was long. She had lost half a stone, experienced pain in her back, legs and feet, felt numbness in her face and other parts of her body, had reduced reflexes in her eye, as well as distorted hearing, critical fatigue and poor concentration. The disease was having an impact on every aspect of her life.
Lyme disease can be difficult to diagnose because many of the symptoms associated with it also occur with other diseases. To complicate things further, current diagnostic tests don’t always detect early Lyme disease, so many patients who have it receive false negative results. If left untreated or diagnosed late, it can result in a myriad of complications that can affect the skin, the nervous system and the musculoskeletal system.
In 2001, there were 268 cases of Lyme disease reported in England and Wales, rising to 872 in 2015, although current estimates put the actual figure at around 3,000. In the United States, 25,000 cases of Lyme disease were reported in 2014, with the total number of people diagnosed estimated at roughly 10 times higher, according to the Centre for Disease Control and Prevention (CDC).
No one knows what is causing the increase in England and Wales, says Matthew Dryden, director of infection at Hampshire Hospitals Trust in Winchester, and at the rare and imported pathogens department, Public Health England (PHE). “It may be because we’re better at diagnosing it, or because there’s a genuine increase,” he says.
Complex symptoms
Lyme disease is a spirochaete infection caused by pathogenic genospecies of the Borrelia burgdorferi sensu lato group. It is transmitted by the bite of an infected tick. Ticks that carry Borrelia are found throughout the UK, and elsewhere in Europe and North America.
European Lyme disease is slightly different to that found in North America, explains Huyshe-Shires, who became chairman of UK advocacy group Lyme Disease Action in 2007 after being forced to retire from her IT job in the NHS. “In the United States there is one main bacteria species of Borrelia, but in Europe there are several more than that.” This results in a slightly different disease presentation, she adds.
“Not all ticks carry [Borrelia]”, says Dryden, “but if the tick is carrying it, and transmits it, then a symptomatic infection may or may not develop.” He explains that the most common manifestation of Lyme disease is the classic erythema migrans (EM) bullseye rash, but the rash can be variable, and not all patients notice a rash. To add further complexity, some patients develop asymptomatic infections.
Some patients, particularly if they haven’t been diagnosed and treated early, go on to develop neurological symptoms
Patients may also experience flu-like symptoms and fatigue, but these tend to get better if treated early with a short course of antibiotics, explains Dryden. However, he adds that “some patients, particularly if they haven’t been diagnosed and treated early, go on to develop neurological symptoms”.
The main complication of Lyme disease in Europe is neuroborreliosis, a disorder of the central nervous system that is seen in about 10–20% of infected individuals, and occurs within around 6–12 weeks of infection[1]
. In the United States, arthritis is a common complication. Lyme disease that is left untreated or treated late can cause persistent symptoms, and this is often referred to as chronic Lyme disease. Symptoms can resemble chronic fatigue syndrome or various neurological conditions, such as multiple sclerosis or arthritis, and complete recovery may not be possible at this stage.
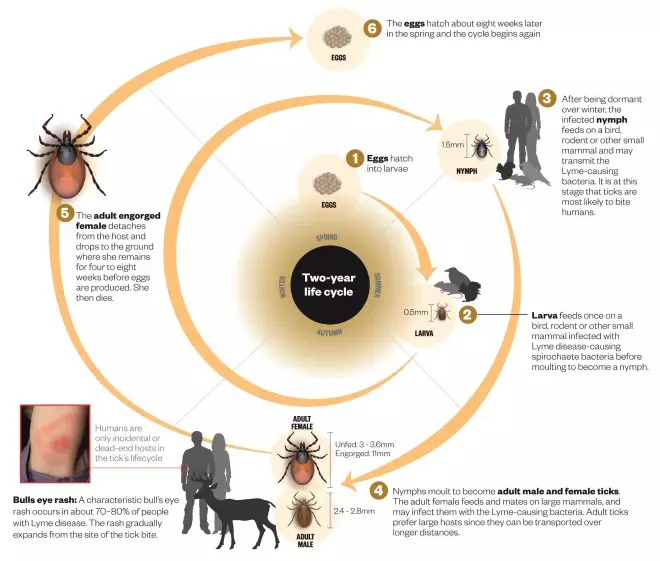
Life cycle of the tick
Source: ECDC, CDC and University of Bristol. Editorial adviser: Matthew Dryden
The tick most likely to bite humans in Britain is the Sheep tick, Ixodes ricinus which, despite its name, feeds from a wide variety of mammals and birds. In the United States, the highest risk comes from the Deer tick, Ixodes scapulars
False negatives and positives
Since early treatment is crucial to prevent complications, accurate diagnosis is essential. Those who have a history of tick bites and a bullseye rash do not need to be tested and should be treated as if they have Lyme disease. However, when the picture is less clear but Lyme disease is suspected, laboratory testing is recommended. False negative results are common in early Lyme disease because the standard test detects antibodies to the Borrelia bacteria and these take several weeks to develop. In addition, some people who receive antibiotics within the first few weeks of a tick bite may not develop antibodies or may only develop them at levels too low to be detected by the test.
In line with this, the NHS Highland National Lyme Borreliosis Testing Laboratory advises that patients with a clinical history consistent with early Lyme disease who have a negative test should be retested six to eight weeks after symptom onset[2]
. It is also possible for the antibody test to give a false positive result, so positive tests are confirmed using a second test, called a Western Blot.
Huyshe-Shires says that because testing isn’t perfect, people who are convinced that they have Lyme “go searching for a positive test”, which has led private laboratories in Germany that are not licensed for diagnosis of Lyme to offer tests to UK patients. Dryden says these private tests “give large amounts of false positivity” and they “have not been adequately validated”. NHS testing in the UK is the same as the conventional validated testing everywhere else in the world, including the United States, Germany and other parts of Europe, he adds.
Treatment options
Different treatments are recommended depending on whether people are diagnosed with early or late Lyme disease (see Table). But for patients with continuing symptoms despite antibiotic treatment, there is disagreement among doctors about whether these symptoms are caused by ongoing infection or damage from past infection, and whether repeated courses of antibiotics will help. Three, small placebo-controlled trials funded by the National Institute of Allergy and Infectious Diseases in the United States have failed to show that prolonged antibiotic therapy is beneficial[3],[4],[5]
.
It’s difficult to justify prolonged courses of antibiotics which can themselves cause harm in the absence of any evidence that there is an active infection
“If there is a consistent and credible history of Lyme exposure, usually most specialists will prescribe a course of antibiotics in symptomatic patients, even if the serology is negative,” says Dryden. However, he points out that if there are no clinical signs and no laboratory abnormalities, “it’s difficult to justify prolonged courses of antibiotics which can themselves cause harm in the absence of any evidence that there is an active infection”.
“But many patients who believe they have chronic Lyme are quite keen to have antibiotics for the long-term,” he adds.
Lack of treatment options for patients who believe they have chronic Lyme has resulted in an entire industry of what Dryden terms “so-called Lyme literate doctors” in the United States, where, he says, a Lyme diagnosis is based on the doctor’s opinion, rather than diagnostic tests. “They treat them with complex regimens of antibiotics, various vitamin supplements, nutritional supplements and sometimes other drugs as well,” he explains, adding that these regimens tend to be prolonged. Although these patients are pleased to be getting treatment, despite paying for it, Dryden says that “there’s very little outcome evidence”.
However, there is one aspect of Lyme disease that experts agree on: the need for more research. US-based advocacy organisation LymeDisease.org is tackling this with the creation of a patient database called MyLyme Data. “The largest study that’s been done in the United States on the [prolonged antibiotic] treatment of Lyme disease had 129 patients[3]
,” explains Lorraine Johnson, chief executive of LymeDisease.org. With more than 3,000 patients enrolled since its launch in mid-November 2015, MyLyme Data hopes to help improve treatment, diagnosis and quality of life for patients who have the disease by pooling data so that research can be performed quickly and inexpensively.
A persistent problem
Kim Lewis, director of the antimicrobial discovery centre at Northeastern University in Boston, Massachusetts, is hoping that research on the bacteria that causes Lyme disease will yield better treatment options for chronic Lyme. “We found that dormant forms of regular cells are what makes it difficult to treat a chronic infection,” says Lewis. These are called persisters, and their dormant state allows them to avoid conventional antibiotic treatment. Lewis is looking for compounds that will be effective against persisters of the Lyme bacteria found in the United States, Borrelia
burgdorferi.

Courtesy of Kim Lewis
Kim Lewis, right, director of the antimicrobial discovery centre at Northeastern University in Boston, Massachusetts, is developing a pulse dosing antibiotic regimen to eradicate chronic stage Lyme disease
In one study, published in 2015, he exposed culture of B. burgdorferi to antibiotics amoxicillin and ceftriaxone[6]
. The dormant persisters were then allowed to wake up for a short time, before the growth medium was hit with a second round of antibiotics. Persisters were “substantially diminished” after four rounds of killing with amoxicillin, and were eradicated below the limit of detection after four rounds of killing with ceftriaxone. The team are now “developing a pulse dosing regimen to eradicate a chronic stage Lyme using a mouse model”. They are also planning a human study, “probably” later in 2016.
Preventing Lyme disease is another option. Despite a vaccine being available for dogs, there is no vaccine currently on sale for human use. This was not always the case. A GSK vaccine, LYMErix, was licensed by the US Food and Drug Administration (FDA) in 1998 but was withdrawn three years later following a series of lawsuits alleging a variety of adverse effects, negative media coverage and subsequent poor sales[7]
. The lawsuits led to an FDA review, phase IV clinical trial, and retrospective study of adverse events, but no compelling evidence was found to show that the vaccine caused these symptoms. The official GSK line for the discontinuation was “poor demand for the product”.
Another vaccine that showed promise was ImuLyme, which was developed by Pasteur Mérieux Connaught, now Sanofi Pasteur. However, this vaccine never reached the market because of technical issues with case reports in the phase III trial, issues related to royalties and patents with GSK, as well as a decision that the market size was likely to be too small to make the vaccine profitable[7]
. Baxter completed a clinical study on a Lyme disease vaccine[8]
, but in 2014, having sold most of its vaccine portfolio, the company stated it was considering “partnering or divesting its R&D development programmes focused on Lyme disease”.
All of these vaccines used the Borrelia species outer surface protein A (OspA) as their foundation, with LYMErix and ImuLyme producing antibodies against the strain most prevalent in the United States, B. burgdorferi. However, Valneva, a company based in Lyon, France, claims to have designed a Lyme vaccine that provides “more protection for a European market” as well as the American market. The vaccine contains OspA fragments from B. burgdorferi, as well as the common European strains B. afzelii, B. garinii, and B. bavariensis
[9]
.
Pär Comstedt, manager of infectious disease models at Valneva, who has worked on the vaccine for the past eight years, says that researchers “have shown efficacy in pre-clinical models”. In 2015, a paper co-authored by Comstedt, stated that mice are protected against both challenge with infected ticks and in vitro grown spirochaetes[10]
. The company is currently working on including more species of Borrelia in its pre-clinical models. Comstedt says it will be a few years until a Lyme vaccine will be on the market, but adds that the vaccine will be “entering clinical development at the end of 2016”.
It is now 15 years since her tick bite, and Huyshe-Shires is still “waiting for the science to catch up”. She continues to have symptoms but has no way of knowing whether they are continuing Lyme disease, or as a result of damage to her nerves. Despite the continued uncertainty in many aspects of the disease, Johnson believes that “we’re right at the brink”. She believes a data and science revolution is happening, which will reinvigorate Lyme disease research. “In the next ten years, we’re going to have good diagnostic tests, and a much better sense of sub-groups and how they respond.”
| Treatment of early and late Lyme disease | |
|---|---|
| Source: NHS Highland National Lyme Borreliosis Testing Laboratory | |
| Early Lyme |
|
| Late or chronic Lyme |
|
References
[1] Rizzoli A, Hauffe HC, Carpi G et al. Lyme borresliosis in Europe. Eurosurveillance 2011;16:19906. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19906
[2] National Lyme Borreliosis Testing Laboratory. User Manual. NHS Highland 2015. Available from: http://www.documents.hps.scot.nhs.uk/labs/strl/lyme-borreliosis-user-manual.pdf
[3] Klempner MS, Hu LT, Evans J et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. New Eng J Med 2001;345:85–92. doi: 10.1056/NEJM200107123450202
[4] Krupp LB, Hyman LG, Grimson R et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 2003;60:1923–1930. doi: 10.1212/01.WNL.0000071227.23769.9E
[5] Fallon BA, Keilp JG, Corbera KM et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d
[6] Sharmaa B, Browna AV, Matlucka NE et al. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrobial Agents and Chemotherapy 2015;59:4616–4624. doi: 10.1128/AAC.00864-15
[7] Poland G. Vaccines against Lyme disease: what happened and what lessons can we learn? C linical Infectious Disease 2011;52:s253–s258. doi: 10.1093/cid/ciq116
[8] Wressnigg N, Pöllabauer EM, Aichinger G et al. Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. The Lancet Infectious Diseases 2013;13:680–689. doi: 10.1016/S1473-3099(13)70110-5
[9] Comstedt P, Hanner M, Schüler W et al. Design and development of a novel vaccine for protection against Lyme Borreliosis. PLOS ONE 2014;9:e113294. doi: 10.1371/journal.pone.0113294
[10] Comstedt P, Hanner M, Schüler W et al. Characterization and optimization of a novel vaccine for protection against Lyme borreliosis. Vaccine 2015;33:5982–5988. doi: 10.1016/j.vaccine.2015.07.095


