
Mclean/Shutterstock.com
When the COVID-19 pandemic came to the UK in March 2020, hospitals had to rapidly adapt to the first wave, redeploying staff, dramatically increasing intensive care capacity and moving outpatient care to remote consultations. Some pharmacists played a vital part in critical care, donning full personal protective equipment (PPE) and working on the frontline. Other members of the pharmacy team may not have been so visible but worked above and beyond to keep services running, taking on new roles and providing expertise. The Pharmaceutical Journal spoke to some of them about what the past year has meant.

Procurement has always been an “unseen service”, says Duncan MacDonald, because staff have usually solved the problem before anyone else becomes aware of it. Since the pandemic, however, there has been a lot more awareness of what we do, he says.
In March 2020, when COVID-19 hit the UK, the work of MacDonald’s team became much more intense, he explains. “The way we would normally work is semi-reactive in that something goes on to backorder, or we receive notification from regional and national teams, and we look at what the issue is. During the pandemic we moved to a much more proactive system.”
His team built a spreadsheet of critical medicines and calculated what would be needed for an average patient on intensive care. The spreadsheet was then populated with real-time numbers, so the team knew exactly how much of each drug would be needed at any one point.
“If we had four days or less of something, we would flag that as part of our command and control structure, so that we could then discuss what to do. Do we need to stop using that and use an alternative, do we need to get emergency aid from somewhere else? Because we were proactive about it, we avoided any kind of total stock outages. But there were days when you were kind of having that panic that it was getting a bit close.”
The medicines on the critical care list evolved over time as treatments, such as dexamethasone and remdesivir, were found to be effective. University Hospitals of Leicester NHS Trust was the top recruiter for the RECOVERY trial, for which the procurement team was responsible for ensuring the right medicines were available. “It was really remarkable. On the same day we used up our last dose of trial stock, somebody else got the first dose outside of trials. That just never happens in research.”
While the work at the start of the first wave was “horrendous”, it was the relentlessness of the second wave that left MacDonald’s team exhausted. “This autumn and winter, if we do have to do that again, we’re well versed in it now — we have the structures and spreadsheets, and we’ll be able to operationalise that quite quickly because we’ve done it twice already. But it will take a toll on people.”

Those working in paediatrics have had a different experience to their colleagues looking after adults during the pandemic. Birmingham Children’s Hospital became a hub for caring for children across the region as other paediatric intensive units were repurposed to care for adults. But, as the nation went into lockdown and outpatient services moved to remote consultations, there were points when Rhian Isaac says she thought, “Where are all the children?”.
While children have been far less susceptible to the worst effects of COVID-19, a small number have developed a significant systemic inflammatory response, termed paediatric multisystem inflammatory syndrome temporally associated with COVID-19, or PIMS-TS. The emerging syndrome, which seems similar to Kawasaki disease, was first reported in May 2020.
Isaac was a member of the regional multidisciplinary team who met daily from that time, providing her expert guidance on treatment for PIMS-TS. “The children became part of the RECOVERY trial, but there were some differences in the drugs that were used [compared with those used in adults],” she explains.
“There were some complicated aspects — immunoglobulins were being used outside NHS England recommendations at one point and remdesivir was given compassionate use for children. There were issues with working out dosing; for example, do we work out dose by age or size? And there were other considerations around getting drugs into children or getting hold of the medicines. Tocilizumab became very difficult to get hold of in the second wave,” she says.
As the pandemic progressed, children with the syndrome were transferred from district general hospitals to Birmingham Children’s Hospital, and logistics became difficult at times. “Something that is really positive is how we have been able to support those hospitals and the improved communication with the pharmacists there. That has been very rewarding.”

The aseptic manufacturing service run by Shakeel Herwitker is based over two sites and produces intravenous infusions and injections, eyedrops, parenteral feeding solutions and chemotherapy. “It was a large operation even before the pandemic, covering a broad range of specialities for our trust and beyond,” he says.
While some services in the hospital closed during the first wave, there was never a day that the aseptic manufacturing site was not operating, he says, with patients totally dependent on the service. But the manufacturing services team also started to think about what more it could do to support those caring for affected patients. The end result was a nationally driven collaboration between NHS manufacturing sites with capability to prepare ready-to-use injectable medicines to take pressure off those on the frontline.
“Through the first wave, response to demand for ready-to-administer medicines was reactive. But [the response was] more focused and effective during further waves of the crisis,” Herwitker explains.
“Our trust focused on supplying fentanyl syringes, and other NHS production units supported the delivery of other critical compounds. We were filling syringes on an industrial scale and we [also had to] source kit that enabled us to do it.”
The team ended up filling at least 400 syringes a day during the most challenging part of the pandemic. It was distributed nationally across the NHS through a wholesaler in Oxford. It is, says Herwitker, refreshing to work collaboratively in this way with other NHS manufacturing sites. It is something that he hopes continues and develops through the implementation of the ‘Transforming NHS pharmacy aseptic services in England’ report, published by the government in autumn 2020.
“NHS production units across the country have demonstrated their capability in responding to a need that has been received very positively by our clinical colleagues and helped to facilitate better patient care.”

As the quality assurance lead for technical services for his trust, Owain Jones oversees the production of medicines in the aseptic unit but is also is in charge of the quality control of medical gases. Throughout the pandemic, it has been the possibility of the hospital oxygen systems failing that has given him sleepless nights. Staff were working around the clock to convert new intensive care wards and every time a new section of pipe was added to deliver oxygen, it was Jones’s job to sign it off.
“We were really concerned about oxygen levels. When you get past a certain point, you have a pressure drop and at that point the system can’t take it and the whole system goes. The engineers were calculating the numbers and doing what we could do to introduce this as safely as possible. You just thought, if it carries on this trajectory, we’re going to hit that point.”
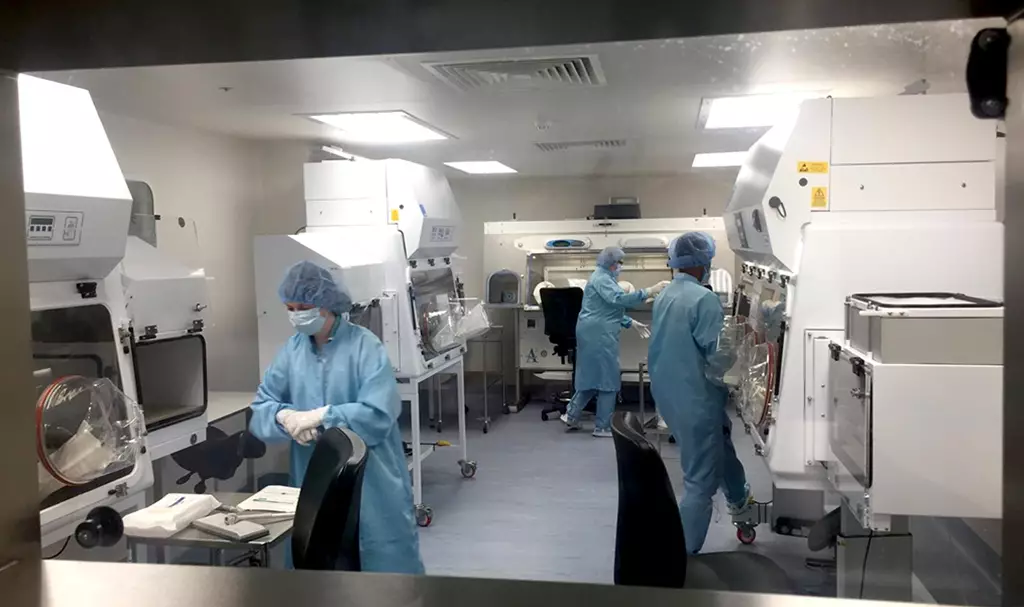
Those first few months, he says, were awful. “It’s never been so stressful, even being removed from the frontline.” Medics were unsure how to treat patients and asking for support with different medicines combinations, before things would suddenly change again.
In March 2020, his team moved as quickly as they could to make up injectable syringes for the nurses on intensive care. “We were particularly badly hit in South Wales. Our nurses were working 12-hour shifts, and at least 4 or 5 hours of those were drawing up medicines. The only area that the nurses, who were in PPE, had was a tiny little bench on the end of the bed. So the risk of making errors or adding some microbial contamination to these products was high,” Jones explains. “When we make batches, [the nurses] can just take them out of the cupboard and hook them up.”
By the time the second wave hit, the technical services team had become more involved in COVID clinical trials. “We were creating this essentially new treatment from a cocktail of monoclonal antibodies. It involved two different vials, and both had clear fluids. They were really awkward volumes as well.”
Unlike most clinical trials, there would be little warning that a medicine was needed, Jones adds. “It was quite a lengthy process; it could take two hours to manufacture and it would take up one of our chemotherapy isolators for that time.”
In the midst of the second wave, the intensive care department moved to a new hospital up the road and the pharmacy team merged into one site. The biggest lesson for Jones has been the importance of collaboration. “We’re naturally risk averse because we have to make things safe but it has pushed us to be more adaptable and move quickly, cutting through the bureaucracy to make a decision.”

Some of the first COVID-19 patients in the UK were treated at the Royal Hallamshire Hospital, where Vicky Goodall is based. At that time, no one really knew what they were dealing with. Each time a patient was transferred to the infectious diseases ward, they were wheeled past Goodall’s office and the door would be locked as an infection control precaution, so that no one would accidentally encounter a COVID-19 patient. But the numbers were increasing to the point where it got ridiculous, she says. Ultimately, extra wards were opened, and patients were also treated in intensive care at the Northern General Hospital in the city.
As the number of patients increased, multiple COVID-19 clinical trials began, and Goodall took on a new role overseeing dissemination of the most up-to-date information on the clinical systems. “We needed to put everything on the electronic prescribing system to make sure that the drugs could be prescribed safely, and the pharmacists knew what it was and what it was being prescribed for,” she says.
Some drugs were being given in unusual doses, or were very niche or being used off license. The use of some drugs changed over time and some were used when they wouldn’t normally be — for example, anticoagulants in patients who didn’t have a clot, she explains.
“Every time something was added in or removed from a trial, we had to update the record. For the RECOVERY trial, I think we’re now on version 8 or 9.” It meant poring over trial protocols and amendments and pulling out the relevant bits to update the systems. The electronic prescribing system became invaluable. “It allowed us to say, for this patient you have this drug and two options for the dose. Some [drugs] have three different doses, depending on renal function, and it made it easy for the doctors and you could dispense using that system as well.”
In all, Goodall has overseen seven trials during the pandemic, including one that has only just launched. “I don’t normally have much to do with trials. It’s been very interesting, and it’s kept me up to date with everything. For an infectious disease pharmacist, this is it — there’s nothing bigger than a global pandemic.”
You may also be interested in
