Paolo Beghini
For more than 40 years, Michael Russell, a professor emeritus of microbiology and immunology at the University at Buffalo, New York, has studied the web of specialised cells and secretions that protect the body’s mucosal surfaces, including the upper respiratory tract. Yet, despite reams of evidence showing how important the mucosal immune system is, it has remained in the shadow of the more familiar systemic immune system — the bloodborne immune cells and antibodies that fight established infections[1].
This could be about to change. In 2020, intramuscular vaccines against SARS-CoV-2 were developed at breakneck speed and were spectacularly effective at reducing the incidence of serious illness relative to infection rates.
However, says Russell, “what they don’t do to any appreciable extent is to prevent the initial acquisition of the virus infection in the upper respiratory tract”.
The systemic and mucosal immune systems operate largely independently of one another. Vaccines injected into the musculature of someone’s upper arm result in the production of circulating antibodies and activated T cells, says Russell, “but these do not, to any great extent, get into the secretions”[2].
The easy vaccines have been done, now we’ve got to get a little bit more sophisticated
Michael Russell, a professor emeritus of microbiology and immunology at the University at Buffalo, New York
Once this is appreciated, the high incidence of breakthrough SARS-CoV-2 infections in people who are vaccinated makes sense, as does the onward transmission of virus following such infections.
“The easy vaccines have been done,” says Russell. “Now we’ve got to get a little bit more sophisticated. And think more carefully about exactly what it is we want to accomplish, and how we’re going to go about that.”
The alternatives increasingly being explored are mucosal vaccines, which target the nasal or gastrointestinal linings to evoke mucosal immune responses that should fight SARS-CoV-2 before it establishes itself in the host[3–5].
“It doesn’t take a lot of antibody to be effective in the mucosa,” says Sean Tucker, founder and chief scientific officer at Vaxart, a Californian biotechnology company developing mucosal vaccines, “and we think that it can block infection a lot better just because it is the first line of defense”.
There are now dozens of mucosal COVID-19 vaccines entering general use, undergoing pivotal trials or moving up the translational chain[6].
Furthest along are those that have recently been approved for use in Iran, Russia, China, and India, while several are in large-scale phase III trials, including one sponsored by the World Health Organization (WHO).
Developers have high hopes for these vaccines, saying they may not only do better at halting infections, but could also supress onward transmission, induce longer lasting immunity or generate immune responses that better protect against multiple variants of SARS-CoV-2.
Equally alluring is the fact that these new agents —which are in the form of tablets, nasal drops or sprays — should be considerably easier to distribute and administer, negating the need for trained medical professionals in dedicated vaccination centres. This would be a massive logistical advantage in high income countries — and potentially transformative in low- and middle-income countries. Especially if the vaccines can be transported and stored in fridges rather than freezers, or, in some cases, at room temperature.
While this sounds great in principle, Benjamin Goldman-Israelow, a clinician and immunologist at Yale University, Connecticut, cautions that “there are a lot of unknowns in the mucosal space”.
Pre-pandemic, only a handful of such vaccines were in routine usage[7], and it made sense, says Goldman-Israelow, for 2020’s huge government-backed vaccine programmes to focus on more conventional intramuscular vaccines.
This view is underscored by certain mucosal vaccines having failed early-stage clinical trials[8,9]. While regulators in some countries have approved mucosal vaccines, there is a paucity of data emerging from those countries; the field awaits a definitive example of a mucosal vaccine protecting against SARS-CoV-2 in the real world.
Two immune systems
The reason for Russell’s interest in mucosal immunity is straightforward: “Most of our infections arise through mucosal surfaces,” he says, “we either eat them, drink them, breathe them, or acquire them through sex”.
The mucosal immune system operates in the linings of the respiratory, gastrointestinal and reproductive tracts, as well in the middle ear and eye, to prevent infections. And around twice as many immune cells protect these external-facing surfaces than function in the circulation.
While vaccines that present antigens at mucosal surfaces can induce systemic immune responses, their major draw is the potential to create an immune memory among the cells that patrol the mucosa.
The cells of both immune systems are made in lymphoid tissues and belong to the same familiar families of cell types, such as T cells and antibody-making B cells. But mucosal immune cells distribute to mucosal sites owing to specific signalling molecules they express[10].
The reason an oral vaccine can potentially protect against an airborne virus, such as SARS-CoV-2, is that the gastrointestinal immune cells that respond to the vaccine recirculate, and those cell lineages populate other mucosal sites, including the nasal epithelium. For example, in 2020, Vaxart showed that a flu vaccine pill it had developed led to nasal immunity and protection against infections[11].
Mucosal immune cells also have certain distinctive functions. Notably, unlike circulating B cells, which secrete monomeric antibodies, mucosal B cells produce secretory immunoglobulin A (IgA), in which two or four individual antibodies are conjoined to produce a layer of protective polymeric antibodies (see Figure). This property of IgA can make it highly effective at binding to and neutralising pathogens, including, it seems, SARS-CoV-2[12].
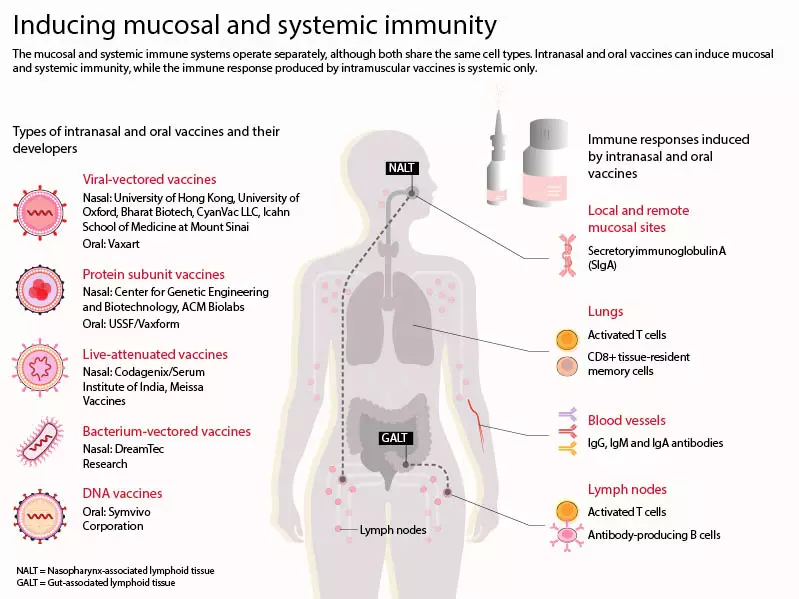
Source: Adapted from Alu et. al
Alisdair Macdonald
But one feature of the mucosal immune system makes developing mucosal vaccines tricky. Conventional intramuscular injection sites are sterile, meaning immune cells there respond robustly to foreign antigens, whereas mucosal surfaces are constantly bombarded by external entities, while also being home to vast populations of commensal microorganisms[13,14].
Consequently, Goldman-Israelow says, the resident mucosal immune system “has to learn not to respond to everything it sees. It has to be able to differentiate bad from not bad”. And so, to create a successful mucosal vaccine, he explains, “you have to overcome that tolerance”.
Whether it is using some form of pro-inflammatory adjuvant or an attenuated live virus — and there is a good deal of variety in this sphere — vaccine developers must ensure that their strategy does indeed generate an adequate immune response.
You can’t create a vaccine where everyone goes into respiratory arrest or everyone’s coughing
Benjamin Goldman-Israelow, a clinician and immunologist at Yale University, Connecticut
On the flipside, such vaccines cannot cause too large a response either. “You can’t create a vaccine where everyone goes into respiratory arrest or everyone’s coughing,” says Goldman-Israelow, adding that this balance is “a difficult needle to thread”.
For instance, in 2000 and 2001, an intranasal flu vaccine led to significant numbers of recipients developing Bell’s palsy, where their faces became temporarily paralysed[15]. This side effect is now thought to have been caused by the adjuvant added to bolster the immune response[16].
The withdrawal of this product contributes to the existence of very few mucosal vaccines in standard usage from which developers can draw lessons. For example, oral vaccines exist for polio, cholera, salmonella and rotavirus, most of which are based on attenuated strains of bacteria or viruses. But Flumist (AstraZeneca), for flu, is the only approved intranasal vaccine — and it is only licensed for use by people aged 2–49 years, owing to its safety and efficacy profile.
Also providing challenges, Russell says, are substantive differences in the biology of mucosal immunity between rodents used in preclinical trials and humans, and the fact that assays of immune cells and antibodies in mucosal secretions are much more technically challenging than those used on blood samples.
Underlining the first point, two companies have seen their intranasal vaccines fail early-stage human trials despite promising preclinical data. In June 2021, the Maryland-based biotechnology company Altimmune halted trials of its intranasal SARS-CoV-2 vaccine. And, in October 2022, researchers at the University of Oxford stopped trials of a reformulation of AstraZeneca’s viral vector COVID-19 vaccine as a nasal spray. In both cases, the vaccines evoked immune responses that were too weak and/or inconsistent to continue the trials.
What’s out there?
The mucosal vaccines out there for COVID-19 — intended as either a primary series or a booster — are at various stages of development. Some of these are reformulations of drugs used already as intramuscular agents, while others have been developed specifically for mucosal targeting.
However, despite 2022 having seen mucosal vaccines launched in several countries, phase III trial data have yet to be published on any of them.
The products launched by Bharat Biotech in Hyderabad, India (used as a two-dose primary series and a booster), and CanSino Biologics in Tianjin, China (used as a booster), have been reported to elevate levels of serum antibodies — in CanSino’s case, better than an intramuscular shot of the same vaccine[17]. Experts note, however, that these companies have not released data directly addressing mucosal immune responses.
Rob Coleman, the chief executive officer of Codagenix — a company based in Farmingdale, New York, that is developing a COVID-19 vaccine given by intranasal droplets — says there needs to be a reckoning on how best to assess the efficacy of mucosal vaccines in early-stage trials, because some are likely to induce less systemic immunity than intramuscular jabs, while still generating consequential mucosal immunity.
One of the silver linings of the pandemic may be forcing regulators to think about other endpoints for mucosal vaccines
Rob Coleman, the chief executive officer of Codagenix
“It’s really on governments, regulators and the scientific community to understand what’s the true way to measure that the mucosal vaccine is working without hanging their hat on the humoral immune response,” he says. “One of the silver linings, if you will, of the pandemic may be forcing the regulators to think about other endpoints for mucosal vaccines.”
Codagenix is a synthetic biology company that makes live-attenuated virus vaccines according to genome sequences designed to be non-virulent by algorithms. Live-attenuated virus vaccines have a long history of success but have not yet been used to target SARS-CoV-2.
The advantage of such vaccines, says Coleman, is that “they mimic a natural infection; you have a weakened version of the virus that gives you all the proteins … and so you make this much broader, more robust immune response”.
When the first human trial participants received Codagenix’s COVID-19 vaccine in spring 2021, Coleman says, very low levels of the vaccine replicated (as expected) and everyone generated a serum antibody response, with 40% having detectable IgA against SARS-CoV-2[18]. To test functional mucosal immunity, the volunteers were given a second nasal dose of the attenuated virus. The acquired immunity stopped it from replicating at all.
The researchers also kept serum from these participants; a year later, they tested the T cells in these samples against the peptide pool of the Omicron variant and saw the T cells respond strongly to the Omicron antigens. “We vaccinated those folks in London,” says Coleman, “and they all made an anti-Omicron cellular immune response before the strain even existed.”
Coleman says these data — plus having partnerships in place for large-scale production and distribution — convinced the WHO to include the vaccine in its ongoing Solidarity trial. In this large-scale trial, involving more than 20,000 people, the vaccine is being used as a primary series across Africa, and potentially soon in South America and Southeast Asia — the primary endpoint being a reduction in infections.
This will provide a large dataset on safety and real-world efficacy, and could lead to WHO approval globally. Coleman says he hopes to get results back in very late 2022 or early 2023. In the meantime, Codagenix is also exploring its vaccine as a booster in a UK trial.
Also planning a study in the UK is Vaxart. Tucker says its COVID-19 vaccine comes as an aspirin-sized tablet that is stable at room temperature, meaning it could be easily stored and distributed worldwide, perhaps even being mailed to people. He also says such vaccines could win over the sizeable number of people who decline vaccines because they are fearful of injections.
Unlike existing oral vaccines, Vaxart’s vaccine uses a non-replicating viral vector that infects gastrointestinal cells, making them express the chosen antigen(s), plus a pro-inflammatory signalling molecule that acts as an adjuvant. To date, it has trialled vaccines against SARS-CoV-2 that contain either just the spike (S) protein or the S protein plus the nucleocapsid (N) protein of the original Wuhan variant, and is now working on Omicron-specific vaccines.
We get a local response in the intestine, but we also are getting distal responses in the saliva and the nose
Sean Tucker, founder and chief scientific officer at Vaxart
In phase I trials in late 2020, the S+N protein vaccine was soundly immunogenic. “We get a local response in the intestine,” Tucker says, “but we also are getting distal responses in the saliva and the nose”.
Taking samples from these trial participants a year later revealed that most of them still had significant mucosal antibodies and that these reacted with all available SARS-CoV-2 variants[19]. Tucker believes that this is likely owing to polymeric IgA potentially being a more cross-reactive immunoglobulin than circulating monomeric ones.
The company also obtained positive data using its pill as a booster[20]. And, next up, is a challenge study, where people who have already received mRNA vaccines will be given Vaxart’s pill or a placebo, before being exposed to SARS-CoV-2 60 days later.
As well as assessing efficacy, the study will allow researchers to characterise mucosal and systemic immune responses, and relate these to protection against infection. “Once we do that,” Tucker says, “we’ll know what’s important from the standpoint for protection from a mucosal vaccine. Because while serum antibodies are a nice correlate for protection, we don’t know if this has to be the case for mucosal vaccine.”
Vaxart has also performed an experiment in animals, showing that hamsters given its mucosal vaccine, then exposed to viral loads big enough to induce a breakthrough infection, were less likely to transmit the virus to other animals than unvaccinated animals were. Though the mechanism is uncertain, it may be that secreted IgA binds to, and neutralises, viral particles before they are expelled[21].

Vaxart
At Yale, Goldman-Israelow — alongside colleague Akiko Iwasaki, sterling professor of immunobiology and molecular, cellular and developmental biology — is working on an intranasal peptide vaccine, designed to act solely as a booster. The approach they are using has so far only been tested in mice, but in these animals, delivering the booster after a single intramuscular dose of an mRNA vaccine provoked robust mucosal immunity that protected them from SARS-CoV-2 infections[22].
The work led Iwasaki to co-found a spin-out company called Xanadu Bio, in February 2022, which, Goldman-Israelow says, will soon start testing this mucosal booster strategy in non-human primates.
Looking back at initiating the project in late 2020, Goldman-Israelow says, “we didn’t think it would necessarily be important for SARS-CoV-2. We were more thinking, ‘What if, for the next pandemic virus that comes around, we were able to have mucosal vaccines available?’”
The maturation of mucosal vaccine platforms could indeed be a boon for tackling future respiratory disease pandemics, but, of course, SARS-CoV-2 has not been conquered. And Goldman-Israelow believes the case for mucosal vaccines is becoming ever stronger.
Coleman concurs: “I think the regulators and the governments around the world […] know that we would be very well off if we had some sort of mucosal approach as a way to stop or actually slow the spread and get in the lead.”
Researchers and developers know, however, that the prioritisation of such vaccines will rest not just on trial results or data emerging from approved vaccines, in countries such as India, but on the political prioritisation of mass public health initiatives on SARS-CoV-2 — and how the virus evolves.
“If there’s a big raging pandemic, it might be easier to get approval than if it kind of disappears,” Tucker says. “My expectation is, there’ll be a new variant that pops up in the next three to six months, and then we’ll all be scrambling. Hopefully, it’s not going to be nasty. But if it is, I think having more than just injected vaccines available is important.”
- This article was updated on 9 December 2022 to clarify who Flumist is licensed for
- 1Russell MW, Moldoveanu Z, Ogra PL, et al. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020;11. doi:10.3389/fimmu.2020.611337
- 2Russell MW, Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front. Immunol. 2022;13. doi:10.3389/fimmu.2022.957107
- 3Alu A, Chen L, Lei H, et al. Intranasal COVID-19 vaccines: From bench to bed. eBioMedicine. 2022;76:103841. doi:10.1016/j.ebiom.2022.103841
- 4Miteva D, Peshevska-Sekulovska M, Snegarova V, et al. Mucosal COVID-19 vaccines: Risks, benefits and control of the pandemic. World J Virol. 2022;11:221–36. doi:10.5501/wjv.v11.i5.221
- 5Waltz E. How nasal-spray vaccines could change the pandemic. Nature. 2022;609:240–2. doi:10.1038/d41586-022-02824-3
- 6COVID-19 vaccine tracker and landscape. World Health Organization. 2022.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 25 Nov 2022).
- 7Miquel-Clopés A, Bentley EG, Stewart JP, et al. Mucosal vaccines and technology. Clinical and Experimental Immunology. 2019;196:205–14. doi:10.1111/cei.13285
- 8Doctor V. Altimmune to halt trials for intranasal COVID-19 vaccine. PharmaLive.com. 2021.https://www.pharmalive.com/altimmune-to-halt-trials-for-intranasal-covid-19-vaccine/ (accessed 25 Nov 2022).
- 9Madhavan M, Ritchie AJ, Aboagye J, et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. eBioMedicine. 2022;85:104298. doi:10.1016/j.ebiom.2022.104298
- 10McGhee JR, Fujihashi K. Inside the Mucosal Immune System. PLoS Biol. 2012;10:e1001397. doi:10.1371/journal.pbio.1001397
- 11Liebowitz D, Gottlieb K, Kolhatkar NS, et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. The Lancet Infectious Diseases. 2020;20:435–44. doi:10.1016/s1473-3099(19)30584-5
- 12Wang Z, Lorenzi JCC, Muecksch F, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13. doi:10.1126/scitranslmed.abf1555
- 13Li M, Wang Y, Sun Y, et al. Mucosal vaccines: Strategies and challenges. Immunology Letters. 2020;217:116–25. doi:10.1016/j.imlet.2019.10.013
- 14Mouro V, Fischer A. Dealing with a mucosal viral pandemic: lessons from COVID-19 vaccines. Mucosal Immunol. 2022;15:584–94. doi:10.1038/s41385-022-00517-8
- 15Mutsch M, Zhou W, Rhodes P, et al. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi:10.1056/nejmoa030595
- 16Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Human Vaccines & Immunotherapeutics. 2018;14:550–64. doi:10.1080/21645515.2017.1415684
- 17Wu S, Huang J, Zhang Z, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. The Lancet Infectious Diseases. 2021;21:1654–64. doi:10.1016/s1473-3099(21)00396-0
- 18Codagenix Intranasal COVID-19 Vaccine Shows Potent Cellular Immune Response Against Conserved Viral Proteins, Indicating Potential for Immunogenicity Against Omicron and Future Variants in Phase 1 Data. Codagenix . 2022.https://codagenix.com/codagenix-intranasal-covid-19-vaccine-shows-potent-cellular-immune-response-against-conserved-viral-proteins-indicating-potential-for-immunogenicity-against-omicron-and-future-variants-in-phase-1-dat/?pag=1 (accessed 25 Nov 2022).
- 19Vaxart Publishes Clinical Data Suggesting its Oral COVID-19 Pill Vaccine Candidate Induces Long-Lasting Mucosal Immune Responses that are Highly Cross-Reactive. Vaxart. 2022.https://investors.vaxart.com/news-releases/news-release-details/vaxart-publishes-clinical-data-suggesting-its-oral-covid-19-pill (accessed 25 Nov 2022).
- 20Vaxart Announces Positive Top-line Phase II Clinical Study Data Demonstrating Safety and Immunogenicity of Its Wuhan S-Only COVID-19 Pill Vaccine Candidate. Vaxart. 2022.https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-top-line-phase-ii-clinical-study-data (accessed 25 Nov 2022).
- 21Langel SN, Johnson S, Martinez CI, et al. Oral and intranasal Ad5 SARS-CoV-2 vaccines decrease disease and viral transmission in a golden hamster model. 2021. doi:10.1101/2021.10.03.462919
- 22Mao T, Israelow B, Peña-Hernández MA, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378. doi:10.1126/science.abo2523