Download the full print version of the infographic here.
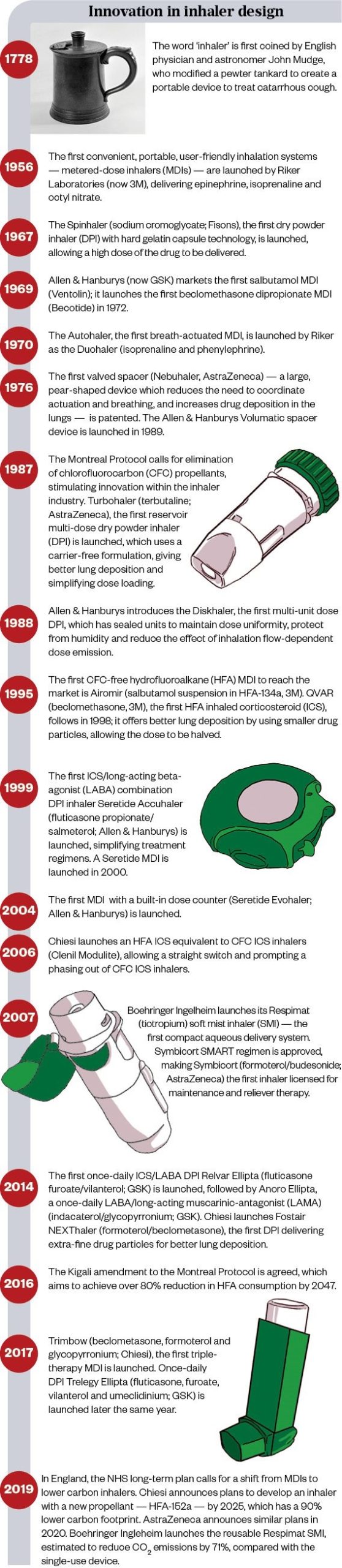
Timeline: Innovation in inhaler design
Source: Mclean
What are the main differences between inhalers?
In adults, there is no difference in clinical effectiveness between a metered-dose inhaler (MDI) with a spacer and a dry powder inhaler (DPI) or soft-mist inhaler (SMI). The most important factor in choosing a device is whether the patient can use it effectively and is happy to do so, followed by cost and environmental impact.
Aerosols
Metered-dose inhalers
- Design: Pressurised canister containing drug, propellants, surfactants, preservatives, and flavouring agents, released through a metering valve and stem when actuated. Can also be breath-actuated.
- Technique: Slow, steady inhalation.
- Advantages: Portable and compact; quick to use; over 100 doses; no contamination of contents; high-dose reproducibility; relatively cheap.
- Disadvantages: Contain propellants; coordination of breathing and actuation needed (if not a breath-actuated device); low lung deposition (10–20%); upper limit to unit dose content; number of remaining doses is difficult to determine; potential for abuse; not all medicines available.
- CO
2
equivalent: High (approximately 10–25kg per inhaler [e.g. generic salbutamol, Salamol, AirSalb, Clenil, QVAR, generic beclomethasone, Seretide Evohaler, Fostair, Sirdupla, AirFluSal, Serevent Evohaler and generic salmeterol]) to very high (approximately 25kg per inhaler or more [e.g. Ventolin Evohaler, Flutiform and Symbicort]).
Soft-mist inhalers
- Design: An extremely fine nozzle atomises the drug solution using mechanical energy imparted by a spring, producing a fine, slow-moving mist.
- Technique: Slow, steady inhalation.
- Advantages: Propellant not required; compact and portable; multi-dose device; high lung deposition (>50%).
- Disadvantages: Only two medicines available; some coordination of actuation and breathing required; some patients may find loading difficult.
- CO
2
equivalent: Low (approximately 1kg per inhaler).
Dry powder inhalers
- Design: Inhalation creates turbulent pressure that deaggregates the drug from the excipient in the dry powder formulation. The clinically effective inspiratory flow rate (IFR) for all DPIs is 30–90L/min, but the IFR for optimal delivery varies depending on the device’s resistance.
- Technique: Quick, deep inhalation.
- Advantages: Coordination between actuation and breathing is not required; propellant not required; small and portable; quick to use; higher lung deposition than MDIs (15–40%); dose counters in most newer designs.
- Disadvantages: May require moderate to high inspiratory flow; some units are single dose; can result in high pharyngeal deposition; not all medicines available.
- CO2 equivalent: Low (approximately 1kg per inhaler).
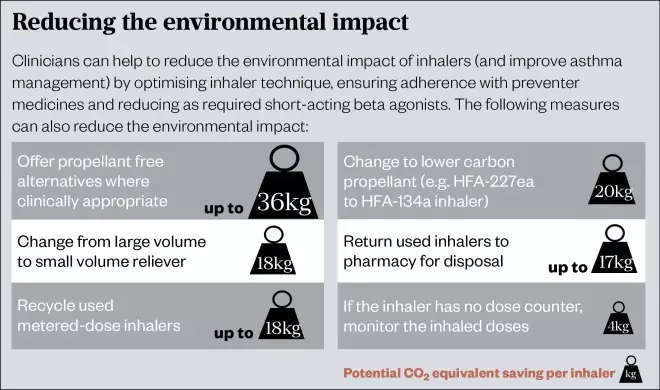
Source: Mclean
References
Sources: Scottish Intercollegiate Guidelines Network/British Thoracic Society, Green Inhaler, National Institute for Health and Care Excellence, Drug Tariff, NHS Business Services Authority, BMJ Open, Multidisciplinary Respiratory Medicine.
Editorial advisers: Anna Murphy, consultant respiratory pharmacist at University Hospitals of Leicester NHS Trust; Toby Capstick, consultant pharmacist, respiratory medicine, Leeds Teaching Hospitals NHS Trust and chair of the UKCPA respiratory group.
Illustrations: Courtesy of Astrazeneca; Javier Trigo; Mclean