To view the infographic, click here
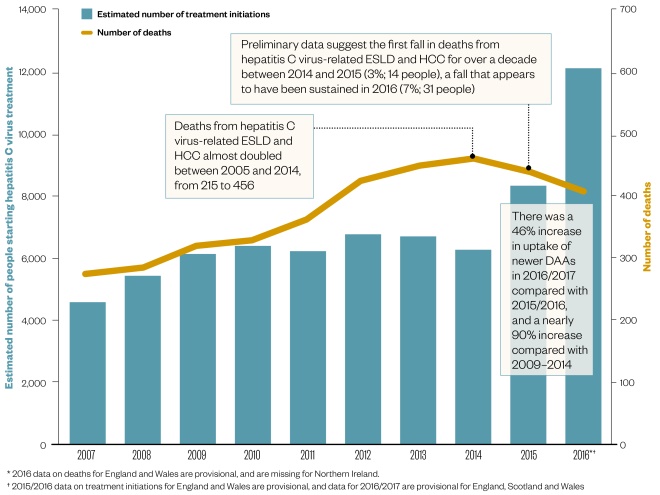
Direct-acting hepatitis C antivirals begin to have an impact
Following the introduction of newer, all oral, direct-acting antivirals (DAAs) in 2014, the number of patients starting treatment has increased by almost 90%, which may be responsible for the fall in end-stage liver disease (ESLD)- and hepatocellular carcinoma (HCC)-related deaths seen over the past two years.
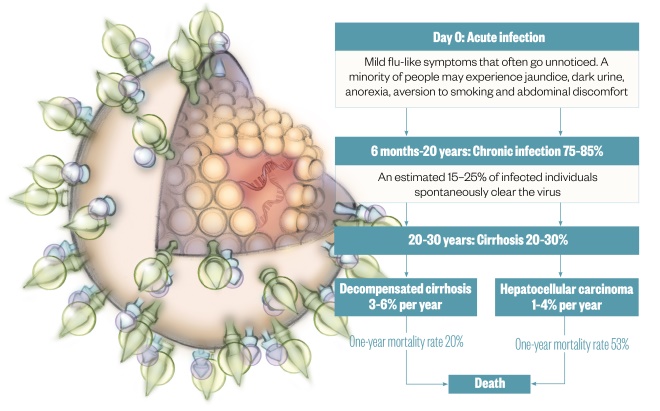
Natural history of hepatitis C
Illustration by Juliet Percival
Hepatitis C is known as the “silent killer” because most people with the disease do not know they are infected until symptoms develop 20–30 years later
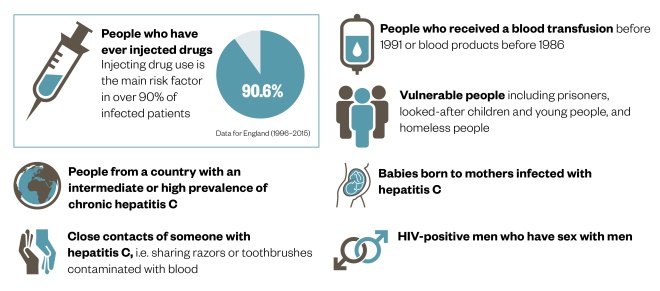
Identifying those at risk of hepatitis C
Hepatitis C is a blood-borne virus that is most commonly transmitted during injecting drug use, but other groups are also at risk.
Timeline: From discovery to cure
1989: Hepatitis C virus is identified as the cause of non-A, non-B hepatitis
1990: A hepatitis C test is developed to detect antibodies in the blood but it is not 100% reliable because it can take up to three months to develop antibodies after infection
1991: Screening of donated blood, blood products, organs and tissues for hepatitis C is introduced in the NHS
1997: The first alpha interferon, Schering-Plough’s Intron A (interferon alpha-2B), is approved for treatment of hepatitis C in the UK
1999: European blood services begin screening blood with nucleic acid amplification testing (NAT), because it detects tiny hepatitis C virus RNA particles
2000: Peglyated interferon (PEGIntron; Schering Plough) is approved for treatment of hepatitis C in Europe
2001: The first combination of pegylated interferon injection and oral ribavirin is approved in Europe for treatment of all genotypes. Treatment lasts 24–48 weeks and has around a 50% cure rate in genotype 1 and 75% in genotypes 2/3
2007: The World Hepatitis Alliance is founded and launches the first world hepatitis day in July the following year
2009: The first rapid antibody test OraQuick (OraSure Technologies), which gives results in 20 minutes, receives a CE mark allowing sale in Europe
2011: The first two direct-acting antivirals (DAAs), boceprevir (Victrelis; MSD) and telaprevir (Incivek/Incivo; Vertex/Johnson & Johnson), are approved in Europe in combination with peginterferon alpha and ribavirin for genotype 1-infected patients. They improve cure rates to about 70% but worsen side effects. Treatment lasts 24–48 weeks
2014:
- Second-generation DAAs sofosbuvir (Sovaldi; Gilead), simeprevir (Olysio; Janssen-Cilag), daclatasvir (Daklinza; Bristol-Myers Squibb) and ledipasvir/sofosbuvir (Harvoni; Gilead) are approved in Europe allowing interferon-free regimens for various genotypes. Treatment usually lasts 12–24 weeks, and is effective in an average of 90% of patients
- Vertex discontinues telaprevir because of competition from newer DAAs
2015: Ombitasvir/paritaprevir/ritonavir (Viekirax; AbbVie), in combination with other medicines, is approved in Europe for treatment of patients infected with genotypes 1 and 4. Dasabuvir (Exviera; AbbVie), in combination with Viekirax, is also approved for genotype 1
2016:
- A combination of velpatasvir and sofosbuvir (Epclusa; Gilead), the first single treatment licensed for all genotypes, is approved in Europe, along with a combination of elbasvir and grazoprevir (Zepatier; MSD) for genotypes 1 and 4
- MSD discontinues boceprevir because of the introduction of Zepatier
- The World Health Assembly approves a global strategy to achieve elimination of hepatitis C as a public health threat by 2030. To do this, and starting from the 2015 baseline, countries and regions need to reduce new infections by 90% and reduce deaths by 65% by 2030
2017:
- Combinations of sofosbuvir, velpatasvir and voxilaprevir (Vosevi; Gilead), and glecaprevir and pibrentasvir (Maviret; AbbVie) are approved in Europe for treatment of all genotypes
- Johnson & Johnson’s Janssen and MSD announce they are halting development of new hepatitis C treatments because of the growing number of treatment options
References
Sources: Public Health England, International Journal of Medical Sciences 2006;3:47, National Institute for Health and Care Excellence, Journal of Hepatology 2014;61:S58, Department of Health, Medicines and Healthcare products Regulatory Agency, European Medicines Agency, Journal of Clinical Oncology 2009;27:1485, Liver International 2012;32:1407.
Editorial advisers: Andrew Radley, consultant in public health pharmacy, NHS Tayside; Ryan Buchanan, hepatology research fellow, University of Southampton; Sarah Sawieres, senior clinical pharmacist —liver and private patients, King’s College Hospital.
Illustration: Juliet Percival.
You may also be interested in
Genomics surveillance programme for hepatitis C launched by UK Health Security Agency

How we treated more than 100 hepatitis C patients at pharmacist-led clinics in North Wales