Pain is a complication of surgical procedures. It is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”.
Post-operative pain occurs because damage to tissues during surgery causes bradykinin, leukotrienes, substance P and prostaglandins to be released. These substances activate the nociceptors (pain receptors) and trigger an action potential in the peripheral nerves (mainly the A› and C afferent fibres)1 which is transmitted to the dorsal horn of the spinal cord and to the brain. Neurotransmitters (prostaglandins and substance P) are involved in transmitting the impulse in the central nervous system, which results in a sensory and emotional response.
Pain following surgery is generally acute, lasting for approximately three days depending on, for example, the surgical procedure. Pain distresses patients. It can also reduce mobilisation, which can slow recovery from surgery. Acute pain can also progress into chronic pain, which is debilitating, costly and difficult to treat. To prevent all this, it is essential to optimise analgesia. This involves regularly monitoring pain (for example, using pain charts) and continually adjusting prescribing as necessary.
Overview of analgesics
There are numerous drugs and treatments available to manage pain, with several options for the route of administration. Different parts of the pathway that transmit pain to and from the brain can be targeted, and therefore using two or more drugs, each acting at a different point, may give better pain relief. That strategy may also mean that lower doses of drugs can be used, reducing the incidence of side effects.
The drug or combination of drugs used and the route of administration chosen will depend on a variety of factors, including the type of operation the patient has had, their perception of the pain they are in, the analgesics (if any) that have already been tried, the efficacy and side effects of the analgesics under consideration and any contraindications to the drug(s) or route.
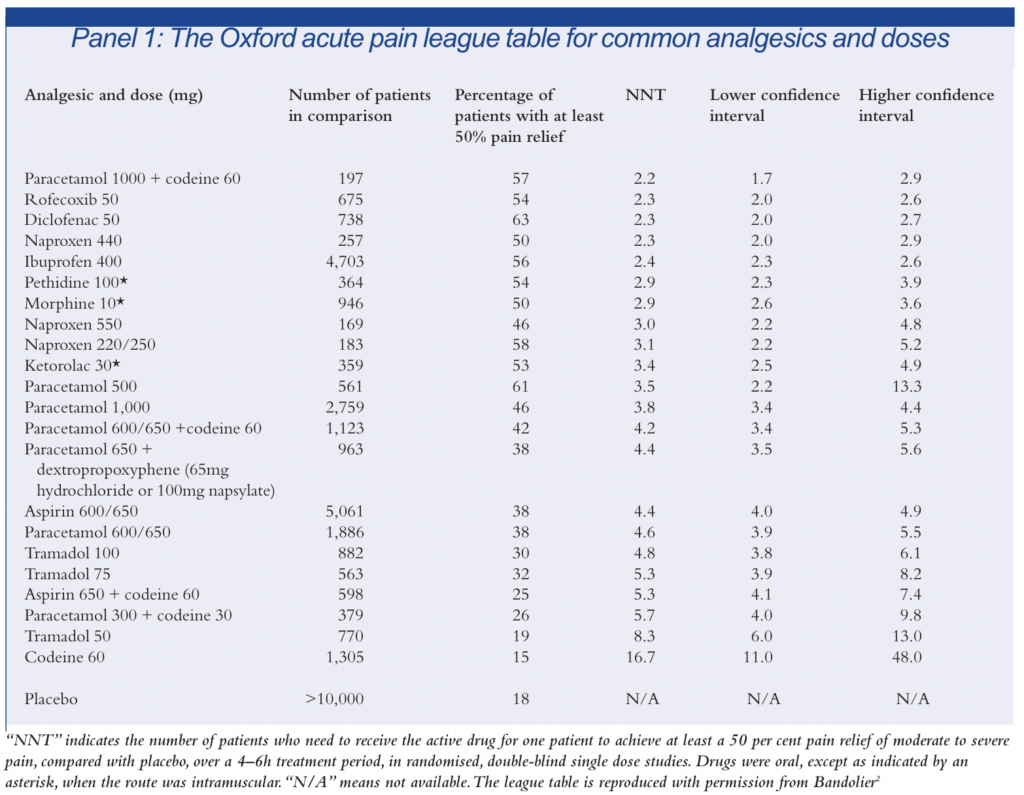
A large amount of work has been done over recent years compiling data from acute pain trials to establish and update a “league table” of analgesics.The Oxford league table of analgesic efficacy (see Panel 1) expresses the efficacy of analgesics in terms of NNTs (number needed to treat), that is, the number of patients who need to receive the active drug for one patient to achieve at least a 50 per cent relief of moderate to severe pain compared with placebo over a 4–6h treatment period in randomised, double-blind single dose studies.2 The perfect NNT is therefore 1, meaning that everyone gets pain relief from the drug, and no one gets pain relief with placebo. NNTs of between 2 and 5 are generally recognised as denoting effective therapies. It should be noted that the Oxford league table considers only the efficacy of analgesics (ie, there is no consideration of side effects or costs.) For some agents, (particularly some of the newer cyclo-oxygenase [COX]-2 inhibitors) not enough data are yet available for meaningful comparisons to be made.2 Information on various analgesics is set out below.
Paracetamol
Paracetamol is one of the most commonly used non-narcotic analgesic-antipyretic agents.3 Its exact mechanism of action is unclear, but involves reducing the amount of prostaglandins synthesised. It has a good side effects profile and is generally considered to be a safe drug, except in overdose. Paracetamol is used frequently for postoperative pain relief. It is the drug of first choice to provide baseline analgesia either alone or in combination with non-steroidal anti inflammatory drugs (NSAIDs) and opioids.
NSAIDs
NSAIDs reduce the amount of prostaglandins by inhibiting COX, which would otherwise catalyse their production. As well as causing pain, prostaglandins have cytoprotective effects in the gastric mucosa, and so inhibiting them can cause gastrointestinal irritation.
Several “traditional” NSAIDs are licensed for post-operative pain. The NSAID of choice is usually diclofenac because it combines efficacy with a reasonable safety profile.4 Studies suggest that it has a lower incidence of gastrointestinal side effects than, for example, piroxicam or azapropazone5,6 (although azapropazone is not licensed for post-operative pain). It is also available in a large variety of formulations, including tablets, capsules, dispersible tablets, slow release tablets, suppositories and injections.4
Piroxicam “melt” formulations are also used in the initial post-operative period. Because they dissolve on the tongue, they are especially useful for patients who can only consume a small amount of liquid following surgery. It is, however, important to note that melt preparations are fast dissolving tablet formulations and are not absorbed sublingually. This means that they can only be used in patients with normal stomach and intestine motility. Piroxicam has a half life of greater than 24h and therefore provides more consistent analgesia than, for example, diclofenac. Some of the newer COX-II selective NSAIDs (rofecoxib and parecoxib) have recently been licensed for the relief of acute pain. The efficacy of rofecoxib is similar to that of diclofenac (see Panel 1) and rofecoxib has the advantage of an analgesic effect that lasts for 24h with once daily dosing.2,7 These agents are more expensive than standard NSAIDs, and so their current role in the management of post-operative pain relief has generally been limited.
NSAIDs are usually only suitable for mild to moderate pain. However, if used in conjuction with opioids, they may reduce the amount of opioids used to treat postoperative pain.8 For this reason NSAIDs are often used in combination with all other forms of opioid analgesia, including epidurals and patient-controlled analgesia (PCA).
Opioids
Opioid analgesics mimic endogenous opioid peptides by causing a prolonged activation of opioid receptors. This (via substance P blockade) reduces the activity of the dorsal horn relay neurones, causing analgesia, respiratory depression, euphoria and sedation. Activation of opiate receptors in the nerve plexus in the gut results in constipation.
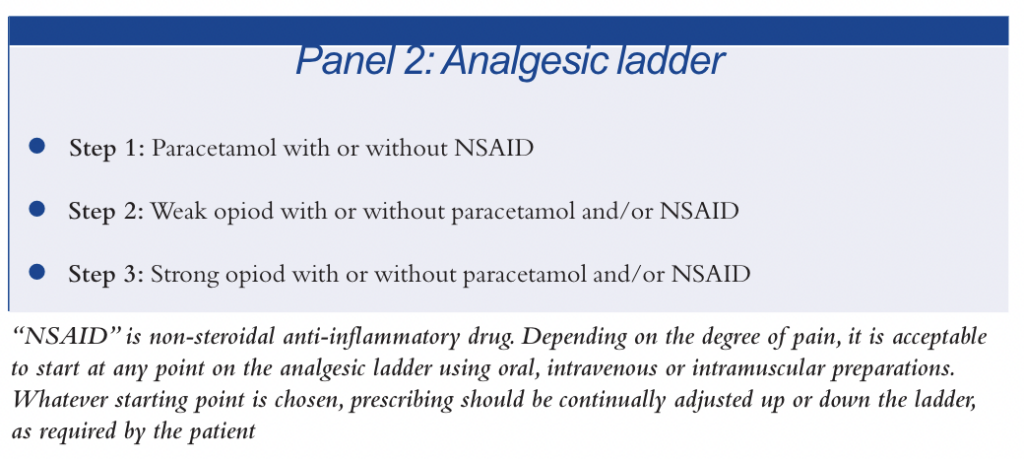
Selecting an opioid for use in acute pain should be done in a stepwise approach, using the “analgesic ladder” (see Panel 2). Whatever starting point on the ladder is chosen, prescribing should be continually adjusted up or down the ladder, as required by the patient.
Codeine and dihydrocodeine
Codeine and dihydrocodeine are both weak opioid analgesics used in mild to moderate pain. Codeine is structurely related to morphine and it has been suggested that its analgesic activity is solely due to the fact that it is metabolised to morphine.
Dihydrocodeine is a synthetic compound with very similar properties to morphine.9 These weaker opioids have about one 12th of the analgesic potency of morphine.10 Codeine or dihydrocodeine are often precribed alone or in combination with paracetamol or NSAIDs for the relief of mild to moderate post-operative pain.
Dextropropoxyphene
Dextropropoxyphene can be prescribed alone or in combination with other analgesics such as paracetamol (co-proxamol). There are few data on its therapeutic value, and at least one major review has concluded that its analgesic effects are less than aspirin and barely more than placebo.11 It is in the same chemical class as methadone. Dextropropoxyphene interacts unpredictably with a number of drugs, including warfarin, and therefore is not usually the first line choice.
Tramadol
Tramadol is a centrally acting analgesic that has opioid agonist activity and is claimed to have other modes of action. Tramadol is generally used for mild to moderate post-operative pain. Tramadol should be used with caution in epileptic patients as it may induce seizures.12
Morphine
Morphine is the usual therapy given for severe post-operative pain. Beliefs that other opioids act faster, last longer or have a better balance between effect and adverse effect for a particular patient often have little empirical credibility.13
Fentanyl
Fentanyl is used in moderate to severe post-operative pain, generally in epidurals, in combination with a local anaesthetic agent.
Anaesthetics and others
Bupivicaine
As a local anaesthetic agent, bupivicaine blocks sodium channels along the nerve fibre, resulting in a local block of pain transmission.For post-operative pain, it is generally used in an epidural (see p449) in combination with an opioid. The degree of epidural blockade depends on the total
amount of drug instilled, which in turn depends on the solution concentration, and the rate of infusion.
Ketamine
Ketamine in high doses is an anaesthetic, but subanaesthetic doses are strongly analgesic. Ketamine has a complex pharmacology. Its analgesic properties may be due to a reduction in nociceptive information traffic in ascending excitatory amino acid pathways linking spinal cord, brainstem and thalamus. Given alone, post-operatively ketamine in analgesic doses causes dysphoria, disorientation and delusions. Low dose ketamine in combination with a moderate dose of opioid can control severe postoperative pain which may have been otherwise unresponsive.9
Nefopam
Nefopam is a unique drug that is structurally related to the antihistamine diphenhydramine, although it does not have antihistamine properties itself. Its mode of action is not fully understood. There is some controversy over its use as a clinical analgesic and it would therefore usually only be used as an alternative agent if pain relief was not obtained from standard analgesics. It has a variety of side effects, including anxiety, sweating, dry mouth and
blurred vision.9 It is not sedative and has little depressant effect on the respiratory or cardiovascular system.
Anticonvulsant drugs
Anticonvulsant drugs have been used in pain management since the 1960s, mainly for the relief of trigeminal neuralgia. Anticonvulsants are also prescribed as adjuvant drugs in other pain syndromes,and in combination with antidepressants, for example, in the treatment of post-herpetic neuralgia.14 These agents may be useful adjuvants for post-operative pain relief, particularly following vascular surgery.
Administration route
Oral administration is now generally the route of choice for pain relief, particularly for mild to moderate pain. It was previously believed that medicines given orally before or immediately after general anaesthesia would not be effective and would complicate surgery, but this is no longer thought to be true in the majority of cases.
Giving analgesics by the oral route is preferred because it avoids the risks associated with injections (for example, risk of infection, risk of pain or hypersensitivity at injection site) and takes up less nursing time. In particular, for NSAIDs, pain relief by the oral route is generally considered to be as effective (and as fast) as using injections or rectal preparations of the same drug at the same dose,15,16 providing the patient can tolerate oral therapy.
For oral analgesia to be effective, however, the drug must reach the stomach and then the small intestine so that absorption can occur. For full absorption,the motility of the stomach and small intestine must be normal. After abdominal or gastric surgery normal stomach motility may not be restored for 15h or more.17 During this period intravenous, epidural and intramuscular (IM) routes are useful because they do not require gastrointestinal motility to be normal. Rectal administration (for example, of paracetamol) can also be considered. Rectal use should be agreed with the patient and approved by the surgeon if rectal administration would affect any anastamoses (surgically made joins between tissues).
Injections of analgesics (usually opioids) are often also necessary where the patient is in severe pain or has undergone particularly complicated or protracted surgery. Options include having a fixed or “as required” regimen controlled by nursing staff. Alternatively, the patient could control an “as required” regimen as part of a PCA system or opioids could be given epidurally as regional analgesia and more recently as patient-controlled epidural analgesia (PCEA).
Controlled fixed dose regimens
It is difficult to decide on an appropriate dose and frequency for each individual patient. Numerous factors influence the amount and frequency of analgesia required, for example: age, weight, height, sex and type of operation. Many factors also influence the kinetics of an IM dose in a patient after surgery, such as the patient’s temperature and circulating blood volume. A patient who returns to the ward hypothermic after a lengthy procedure and hypovolaemic because of inadequate fluid replacement will have poor perfusion of skeletal muscle, resulting in the poor absorption of the opioid analgesia administered. If an increased amount of opioid analgesic is prescribed to overcome this situation and provide an adequate level of pain relief, there is a serious risk that as the contributing factors to the poor skeletal muscle perfusion are corrected, the patient will become “overdosed” with opioid. This may increase the risk of respiratory depression and sedation.18 “As required” or PCA is therefore
preferred.
“As required” regimens
“As required”regimens involve the prescription being written to allow the patient to request a dose from a nurse, when they believe that they need it. If managed well, “as required” regimens mimic the analgesia provided by PCA. However, staffing shortages, ward distractions and controlled drug legislation may limit administration. With “as required” doses, patients may be reticent to ask for an injection, either because they do not want to bother the nursing staff or wish to avoid having an injection, resulting in inadequate levels of analgesia. In these circumstances, infusion devices, epidurals or PCA provide better analgesia and therefore aid recovery.18
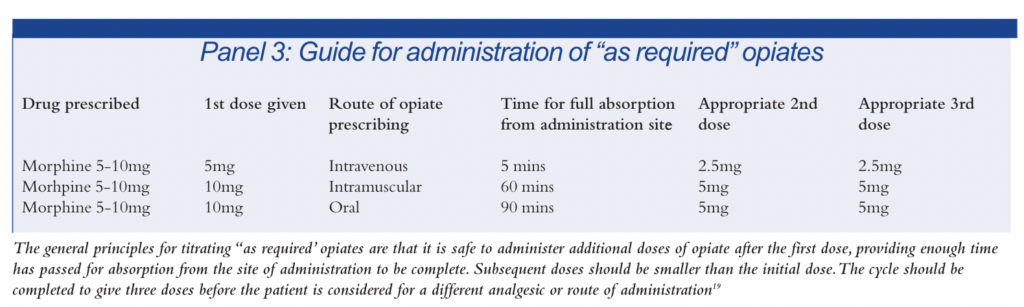
Another difficulty with an “as required” system is in relation to the education and training of the nursing and medical staff in the administration of doses. If a patient requests a drug too often, staff might withhold doses because they fear that the patient will be given an overdose or develop an addiction. Neither of these events is particularly likely, providing the opiate is being used to treat acute pain and is titrated appropriately.18 Panel 3 provides a guide to titrating opiates in an “as required” system. The general principles are that after the first dose it is safe to administer additional doses of opiate, providing enough time has passed for absorption from the site of administration to be complete. Subsequent doses should be smaller than the initial dose. The cycle
should be completed to give three doses before the patient is considered for a different analgesic or route of administration.19
Patient-controlled analgesia
PCA is a method of pain control whereby patients self-administer small doses of an intravenous opioid analgesic using a specially designed pump. This method of opioid administration is generally accepted as safe and effective. In contrast to conventional IM opioid analgesia, PCA is associated with fewer adverse effects, better pulmonary recovery after abdominal surgery, decreased nursing time for drug administration, improved individualisation of drug dosages, improved analgesia and reduced length of stay in hospital. It also allows patients to control their own pain relief.
There are many devices available to administer opioid analgesia via PCA, but they all work on the same principle of allowing the operator to programme them to deliver a set volume of analgesic with a lock out period, during which time the patient can receive no further doses of analgesic. A PCA is not appropriate for all individuals. Patients should be reviewed for their suitability for the device before they undergo surgery. For example, PCA is not suitable for anxious patients because they tend to push the button more often than required and develop side effects.18
Epidurals
Epidurals are an alternative to PCAs where the patient has had lower limb, spine, abdominal or chest surgery. The formulation used is usually a mixture of a local anaesthetic agent and an opioid which act synergistically so that lower doses of each are required to produce the same analgesia with less adverse effects.20
The most commonly prescribed solution is bupivicaine with fentanyl.A review of the literature suggests that bupivicaine 0.125–0.15 per cent with fentanyl 2µg/ml results in effective pain relief without unduly increasing the side effect profile, compared with either drug used alone.21,22,23
As a result of media attention about epidurals, patients often have concerns about having an epidural inserted. These concerns need to be discussed with the patient and their consent gained. Problems associated with the insertion of epidurals (for example, infection and “wrong route” errors) can be minimised by close monitoring of the patient, ensuring a standardised system of administration and using colour coded, dedicated epidural lines.
The most commonly occurring side effect of an epidural is hypotension, resulting in decreased heart rate and blood pressure. This is sometimes caused by bupivicaine, but is more likely to result from the patient being hypovolaemic. If bupivicaine (rather than hypovolaemia) is the cause, then it can be treated with ephedrine.18 Respiratory depression is uncommon with low dose infusions because the high lipid solubility of fentanyl confines its effect within the spinal cord. If respiratory depression is going to occur, it is most likely to do so within the first hour of initiating the
infusion. It can be reversed with naloxone.
Many hospitals have trials underway using PCEA.The epidural is set up with a background infusion of the epidural solution and the patient controls extra bolus doses into the epidural space using a mechanism similar to a PCA’s. There is evidence to suggest that PCEA is better than continual epidural infusions.24 The amount of drug infused is generally less, because the patient is able to vary the dose as required. This in turn can reduce the incidence of side effects,25 while maintaining effective pain relief.26
Conclusion
For simple surgical procedures, the evidence suggests using oral paracetamol and an opioid with or without an NSAID. For the recently licensed COX-2 selective NSAIDs, more work comparing them with the more traditional NSAIDs needs to be done before their place in regimens to relieve post-operative pain can be established.
For complicated procedures, there is evidence to suggest that using epidurals containing combinations of local anaesthetic and opioid can result in a reduced hospital stay and morbidity. Overall it is important that patients are involved and wherever possible take control of their own pain relief. This can be achieved by the use of opioid PCA and PCEA. The key to successful pain management is education of both the patient and nursing staff to provide optimum relief. There are currently a vast range of drugs and techniques available which need to be titrated to each patient’s individual needs.
Acknowledgements
We thank Anne Cole (critical evaluation pharmacist) and Jacquie Trim (acute pain sister) for their help with this work. We also thank the Pharmaceutical Press for permission to reproduce some work from reference 18.
References
- Walsh D.Pain and its modulation. In TENs clinical applications and related therapy. London: Churchill Livingstone; 1997 p11–23.
- Bandolier extra.Oxford acute pain league table for common analgesics and doses. In: Acute Pain. Oxford: Bandolier Feb 2003.p12. Available at www.jr2.ox.ac.uk/bandoleir and www.ebandolier.com (accessed 15 October 2003).
- Rang HP,Dale MM,Ritter JM. Pharmacology.3rd ed.London: Churchill Livingstone; 1995.
- Eltringham R,Casey W,Durkin M. Post operative recovery and pain relief. London:Springer Verlag; 1998.
- Medicines Control Agency/Committee on Safety of Medicines. Current problems in pharmacovigilance. London: The Agency/Committee 2002;28:5.
- Summary of product characteristics for piroxicam. Available at www.emc.medicines.org.uk/piroxicam (accessed 28 September 2003).
- Summary of product characteristics for rofecoxib. Available at www.emc.medicines.org.uk/rofecoxib (accessed 28 September 2003).
- Kirk RM,Mansfield AO,Cochrane JPS. Clinical surgery in general. 3rd ed. London:Churchill Livingstone;1999.
- Alexander JI,Hill RG.Postoperative pain control. Oxford: Blackwell Science; 1987.
- Neal MJ.Medical pharmacology at a glance. 2nd ed. Oxford: Blackwell Science Ltd; 1992.
- Walker R, Edwards C.Clinical pharmacy and therapeutics. 2nd ed. London: Churchill Livingstone; 1999.
- Medicines Control Agency/Committee on Safety of Medicines.Current problems in pharmacovigilance. London: The Agency/Committee 1996.
- McQuay H.Opioids in pain management.Lancet 1999;353:2229–32.
- Bandolier.Oral tramadol in post operative pain. Oxford: Bandoleir. Available at www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/AP003.html (accessed on 28 September 2003).
- McQuay H, Moore A, Justin D. Treating acute pain in hospital.BMJ 1997;314:1531.
- Popat M.Managing postoperative pain relief. Anaesthes Intensive Care Med 2000;1:13–15.
- Dodson M.The management of postoperative pain. London: Edward Arnold;1985.
- Millen S, Cole A. Cholecystectomy. In: Dodds L.editor.Drugs in Use,3rd edition. Pharmaceutical Press, 2004: 569–90. (In press).
- Goldhill D, Stuinil L. Baillieres best practice and research in clinical anaesthesiology. London: Baillieres Tindall; 2000.
- McQuay H. Epidural analgesics. In Wall P. Melzack R.Textbook of Pain. London: Churchill Livingstone; 1994 pp1025–34.
- Benzon HT Wong CA et al.The effects of low dose bupivicaine on post operative epidural analgesia and thrombelastography. Anaest Analg 1994;79:911–17.
- Godney JA.Side effects of epidural infusions of opioid bupivicaine mixtures. Anaesthesiology 1998;53:1148–55
- Badner NH,Komar WE.Low dose bupivicaine does not improve post operative analgesia. Anaest Analg 1994; 72:337–41
- Silvasti M,Pitkanen M.PCEA versus continuous epidural after total knee arthroscopy. Acta Anaesthesiologica Scandinavica 2001;45:471–6.
- Liu SS,Allen HW,OLsson GL.PCEA with bupivicaine and fentanyl on hospital wards: prospective experience with 1030 surgical patients. Anaesthesiology 1998;88:688–695.
- Komatsu H,Matsumoto S, Mitsuhata H, Abe K,Toriyabe S.Comparison of PCEA with and without a background infusion after gastrectomy. Anaest Analg 1998;87:907–10.


