Eye of Science / Science Photo Library
Multi-drug resistant tuberculosis (MDR-TB) strains — defined as being resistant to treatment with isoniazid and rifampicin — are becoming increasingly prevalent. In 2012 an estimated 450,000 people worldwide had MDR-TB, and it was associated with 170,000 death
s
[1]
.
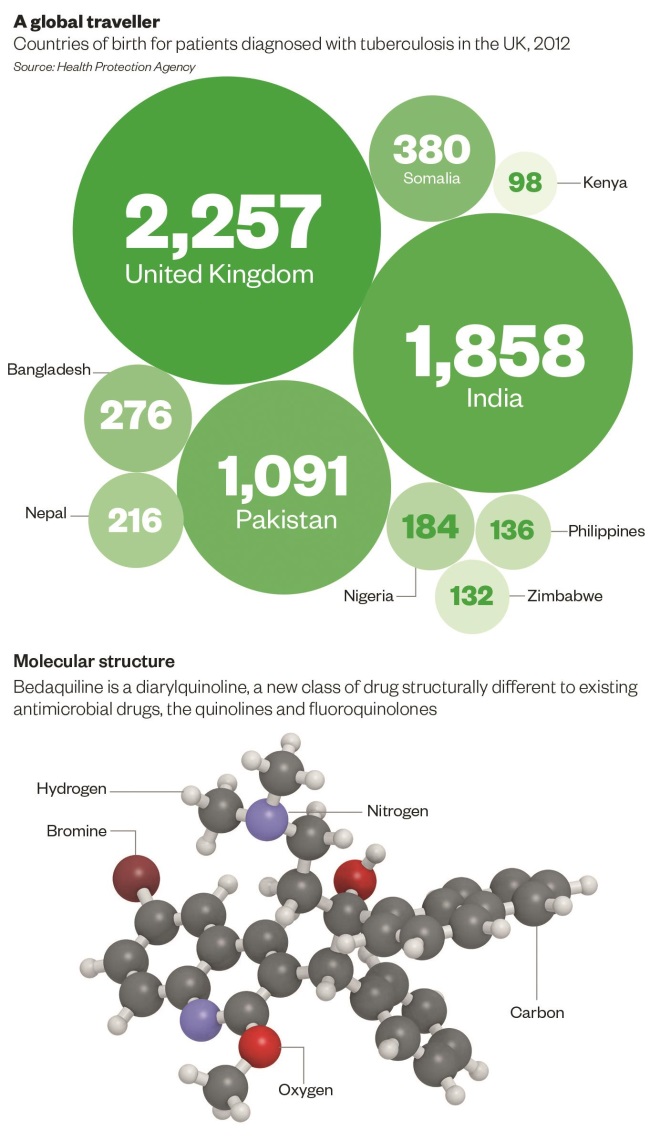
In the UK, 8,751 patients were known to have TB, a prevalence of 13.9 people per 100,000 population. Of these, 73% of patients were born outside the UK, mainly in South Asia or sub-Saharan Africa. Around 1.6% of cases in the UK were MDR-TB, compared with 0.9% of cases in 200
0
[2]
.
Although other antimicrobial medicines are effective against MDR-TB strains, they require treatment for 20 months or longer[3]
and are associated with a high incidence of adverse reactions. This can result in non-adherence, which has contributed to the emergence of extensively drug resistant TB strains.
There is a pressing need for new anti-TB drugs. The first of these new medicines to be launched is bedaquiline (Sirturo), a diarylquinoline. Diarylquinolines differ from quinolones and fluoroquinolones structurally and in their mechanism of action. This means cross-resistance is not predicted to occur[4]
.
In December 2013, bedaquiline received conditional authorisation from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP)[5]
for the treatment of MDR-TB, and it was launched in the UK in June 2014 with a licence for the treatment of pulmonary MDR-TB.
Other tuberculosis medicines in development
Other candidate medicines currently being investigated as tuberculosis treatments include the nitroimidazopyrans, delamanid and PA-824, and a derivative of ethambutol, SQ109. Delamind acquired a UK licence in June 2014, while the other therapies are still in development.
Mechanism of action
Bedaquiline selectively inhibits the enzyme adenosine 5’-triphosphate (ATP) synthase in mycobacteria. This causes microbial death by reducing available ATP supplies
[4]
and creating a pH imbalance (ATP synthase acts as a proton pump)[6]
[7]
. This mechanism of action is entirely distinct from existing medicines, which target bacterial cell walls or nucleic acids.
Bedaquiline is bactericidal against the majority of mycobacterial species, including resistant strains and atypical mycobacteria such as Mycobacterium avium, M. intracellulare
, M. kansasii and M. leprae
[8]
[9]
[10]
. However, it is not effective against Gram positive and Gram negative bacteria, requiring concentrations around 100-fold higher than those used to treat mycobacteria.
Bedaquiline is effective against both active and dormant (non-replicating) TB[11]
. Dormant mycobacteria are highly sensitive to even nanomolar concentrations of the drug, because ATP levels — required to maintain viability in a dormant state — are much lower6,7. This means bedaquiline may be especially effective as a sterilising treatment.
In animal studies, bedaquiline has demonstrated synergy with pyrazinamide[12]
.
Efficacy
Few clinical studies have been conducted into the efficacy of bedaquiline, although two phase II trials have published results.
The first trial demonstrated a delay in early bactericidal activity, a measure often used to assess the efficacy of new medicines. Bedaquiline took four days to achieve a similar reduction in sputum counts to one day of treatment with isoniazid11. This delay is probably related to its unusual mechanism of action, and the disadvantage is offset by its strong sterilising ability and long duration of action.
The second trial assessed bedaquiline’s efficacy against placebo as part of an MDR-TB drug regimen. After eight weeks of treatment negative sputum smears were obtained in 48% of the bedaquiline arm of the study compared with 9% in the placebo arm[13]
. At two years, results also showed a lower incidence of resistance to other components of the regimen for those in the bedaquiline arm[14]
.
Unpublished data from a phase II randomised controlled trial showed a reduction in time to sputum culture conversion (a measure of efficacy) compared with placebo[15]
.
Phase III studies are ongoing.
Administration
Bedaquiline should be.
Bedaquiline is available as 100mg tablets and is initially taken at a dose of 400mg daily for two weeks14 taken with meals, because food doubles its bioavailability15. The dose is then reduced to 200mg three times a week for a further 22 weeks[16]
. This is because of its extremely long half-life (173 hours), which is attributed to gradual redistribution from tissues4,11,16.
The extensive tissue distribution of bedaquiline means a steady state is not achieved for 7–14 days
12, and plasma levels are variable
for at least the first 12 days of treatment
[17]
. Any missed doses during the first two weeks can be ignored, but subsequent missed doses should be taken as soon as possible
15.
Safety
Bedaquiline is generally well tolerated in patients4,11,13. The main side effects are nausea, headache and arthralgia13,14,15. Raised transaminase levels have been reported, so hepatic function should be monitored when treatment is started, and then monthly throughout the course. Treatment should be reviewed if aspartate transaminase (AST) or alanine transaminase (ALT) levels exceed five times the upper limit of normal5,15.
There is evidence of bedaquiline causing a prolonged duration of the QT interval, and patients should have a 12-lead ECG when treatment is started and then at monthly intervals. There are no cases of bedaquiline extending the QT interval by more than 500ms, and it is not considered significant11. However, the effect may be augmented by drugs with similar effects, such as the anti-TB medicine moxifloxacin, and its use may be limited in patients with long QT syndrome.
Serious toxicity is unlikely with bedaquiline because ATP synthase in human mitochondria differs from that in mycobacteria; three larger amino acid substitutions within the binding site sterically prevent bedaquiline from binding to the human enzyme6,[18]
[19]
. Mutations at these sites confer natural resistance to bedaquiline in M. xenopi,
19M. novocastrense and M. shimoidei 8, and account for cases of spontaneous resistance, although the rate of spontaneous resistance is low6 18 [20]
.
Preliminary reports from one clinical trial have raised concerns over an unexplained increase in mortality in the bedaquiline arm compared with controls, with nine deaths (11.4% of participants) compared with two (2.5%). All deaths occurred after completion of therapy, with the majority being disease-related and not linked to QT prolongation. However, the cause of death in four patients has not been established15.
Bedaquiline does not induce or inhibit cytochrome P450 enzymes. However, it is metabolized by CYP3A4 (ref. 16). This means the potential for drug interactions is high given that many commonly co-prescribed medicines are powerful enzyme inducers or inhibitors, such as antifungals and antiretrovirals. Patients should also be advised to avoid alcohol and St John’s wort during treatment15.
Rifampicin was found to halve the availability of bedaquiline in pre-clinical and phase I studies[21]
. Although so far no studies have been conducted to evaluate this interaction, it is advisable to avoid this combination of therapy at present.
No adverse effects have been demonstrated with bedaquiline in combination with efavirenz[22]
nevirapine or lopinavir/ritonavir15. It has yet to be tested with the more potent and widely-used protease inhibitors darunavir and atazanavir.
Place in therapy
Bedaquiline is currently only licensed for pulmonary MDR-TB. In the future it is likely to be reserved for the treatment of MDR-TB, despite also demonstrating equivalent efficacy against drug-sensitive TB and atypical mycobacteria. This is because of its interaction with rifampicin (a mainstay of first-line treatment) and the paucity of potent second-line medicines.
Bedaquiline is a strong sterilising treatment, which makes it a useful substitute for rifampicin, and could be used to shorten the duration of treatment regimens. It has been proposed as a potential candidate for inclusion in once-weekly treatment regimens with rifapentine[23]
.
MDR-TB therapy
Fully-sensitive TB is treated with group 1 drugs. Multi-drug resistant TB is treated with at least five drugs, including as many from group 1 as possible; one from group 2; one from group 3; and two or more from group 4 or group 5 (ref. 24).
The groups are ordered by efficacy, with group 1 the most effective.
Group 1: rifampicin, isoniazid, ethambutol, pyrazinamide, rifabutin
Group 2: aminoglycosides (amikacin, kanamycin), cyclic polypeptides (capreomycin)
Group 3: fluoroquinolones (ofloxacin, levofloxacin, moxifloxacin, gatifloxacin)
Group 4: para-aminosalicylic acid, thioamides (protionamide, ethionamide), cycloserine or terizidone
Group 5: linezolid, clofazamine, clarithromycin, co-amoxiclav, imipenem-cilastatin
Of the group 2 drugs, amikacin is the drug of choice. Capreomycin is used in some centres, despite requiring intra-muscular administration, as there is cohort evidence of lower nephrotoxicity and ototoxicity than with amikacin.
Of the fluoroquinolones, moxifloxacin is most commonly used. Levofloxacin is as effective only in doses over 750mg per day. Gatifloxacin is most potent but is not available in the UK.
References
[2]Health Protection Agency. Tuberculosis in the UK. HPA London:2013.
[4]Andries K, Verhasselt P, Guillemont J et al. A diarylquinoline drug active on ATP synthase of Mycobacterium tuberculosis. Science 2005;307;223-227
[6] Koul A, Vranckx L, Dendouga N et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biologic Chem 2008;283: 25273–25280.
[7] Rao SP, Alonso S, Rand L. et al. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis . Proc Natl Acad Sci USA 2008;105:11945–11950.
[10] Ji B, Chauffour A, Andries K, et al. Bactericidal activities of R207910 and other newer antimicrobial agents against Mycobacterium leprae in mice. Antimicrob Agents Chemother 2006;50:1558–1560.
[15]Janssen-Cilag Ltd. Sirturo (bedaquiline) 100 mg tablets. Summary of Product Characteristics.
You might also be interested in…

Everything you need to know about mpox
Norovirus and strategies for infection control
