Shutterstock.com
There are a wide range of complications commonly associated with cancer (e.g. neutropenia, metastases and alopecia). However, a lesser-known complication is venous thromboembolism (VTE), or, as it has also been termed, cancer-associated thrombosis (CAT). Despite one in five patients with cancer having a VTE during their cancer journey, awareness of the condition in patients and healthcare professionals is low[1],[2]
.
Cancer learning ‘hub’
Pharmacists are playing an increasingly important role in supporting patients with cancer, working within multidisciplinary teams and improving outcomes.
However, in a rapidly evolving field with numbers of new cancer medicines is increasing and the potential for adverse effects, it is now more important than ever for pharmacists to have a solid understanding of the principles of cancer biology, its diagnosis and approaches to treatment and prevention.
This new collection of cancer content, brought to you in partnership with BeOne Medicines, provides access to educational resources that support professional development for improved patient
VTE is an umbrella term that comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). A study in the United States has suggested it occurs for the first time in around 1 in 1,000 people each year[3]
. In addition, CAT accounts for 20–30% of all VTEs[2],[4]
. CAT is an important cause of morbidity and mortality in patients with cancer, with a 2.2-fold increase in mortality, compared with patients with cancer without CAT[5]
.
Studies comparing patients with cancer to those without the disease have also shown that patients with cancer have a four- to seven-fold increased risk of VTE, combined with a two-fold increased risk of bleeding[6]
. CAT is the second leading cause of death, after disease progression, in patients with cancer, and the leading cause of death while taking chemotherapy (i.e. higher than neutropenic sepsis)[7]
. Not all patients who develop CAT are symptomatic, with as many as half diagnosed incidentally following scans[8]
. Patients with CAT are also at an increased risk of recurrence (9.6 per 100 patient years), with the greatest risk of recurrence in the first few months following diagnosis[9],[10]
. To give the figure some context, this rate of recurrence is similar to that observed for male patients, who do not have cancer, diagnosed with unprovoked proximal DVTs, one of the highest risk groups in terms of re-occurrence of VTE[11]
. Patients with cancer have a three-fold increased recurrence risk compared with non-cancer patients, and a higher rate of readmission to hospital because of VTE recurrence within six months of diagnosis (22% for those with cancer and 6.5% for those without)[12]
.
Awareness of CAT has increased in recent years. At the All-Party Parliamentary Thrombosis Group meeting in October 2016, data was published showing that an estimated 4,000 cancer deaths per year in England and Wales may be as a direct result of preventable CAT[1]
. The report highlighted that thrombotic events specifically attributed to CAT are increasing at a higher rate than for total cancer deaths and that the incidence of VTE in cancer may be higher than previously estimated[1]
.
This article examines the pharmacist’s role and summarises the evidence underpinning current guidelines, highlighting best practice points throughout to help improve practice.
The pharmacist’s role
Although understanding of the clinical elements of CAT are well developed, understanding of the patient’s experience remains limited. The PELICAN study, published in 2015, aimed to explore the understanding and experiences of patients with cancer, from the perspective of CAT[13]
. The study highlighted a lack of awareness of the signs and symptoms of CAT in patients with cancer (e.g. patients attributing shortness of breath to being a side effect of chemotherapy, resulting in delayed access to diagnosis) and an unsatisfactory experience in terms of treatment initiation.
The study also identified that patients feel uninformed about their diagnosis, and subsequently feel extremely anxious. One of the key recommendations was the need to raise awareness of the signs and symptoms of CAT, and to develop dedicated CAT pathways[13]
.
A study exploring patient preference of anticoagulant strategies in CAT highlighted that patients place greatest value on factors such as the potential impact of anticoagulants on their chemotherapy and efficacy of treatment[14]
. Preference for oral therapy was deemed to be of moderate importance[14]
. Patient education and the development of patient-specific treatment strategies are of paramount importance in the management of CAT.
Pharmacy teams can manage CAT patients by:
- Advising on the risks of developing CAT — this may be in specialist oncology clinics, or when issuing chemotherapy within community or hospital pharmacies;
- Ensuring appropriate VTE risk assessment and thromboprophylaxis for both admitted and ambulatory patients with cancer;
- Development of CAT pathways, including selection of the most clinically appropriate anticoagulant for the patient (see Figure 1);
- Counselling on new anticoagulant therapies, including expected and unexpected side effects;
- Ensuring safe supply of anticoagulants, including monitoring and dose adjustments;
- Liaising with the wider multidisciplinary team and patients to form patient-specific action plans at the six-month review.
A considerable number of clinical research developments related to CAT have been made in the past few years, yet there is still work to do to improve the patient experience. Data is already emerging that show the impact of dedicated CAT services on patient experience[15]
. There is significant scope for pharmacy to play a vital role in improving the care of this patient group.
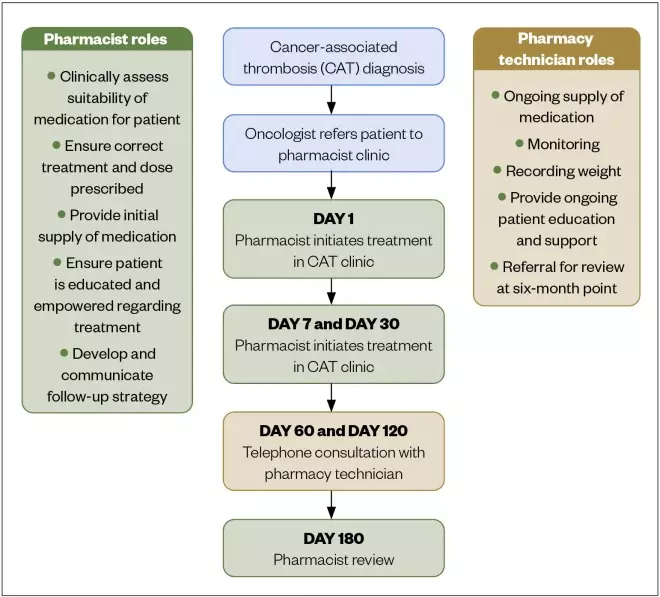
Figure 1: The Singleton Hospital cancer-associated thrombosis pathway
Why does cancer-associated thrombosis occur?
The association between cancer and thrombosis was first described by Bouillard in 1823 and later in 1865 by Trousseau[3],[16],[17],
[18]
. Since then, numerous studies have illustrated this two-way association[7]
. However, the aetiology and pathophysiology of CAT remains unclear, with most literature suggesting a complex interplay between different factors, as opposed to a single mechanism. Rudolf Virchow’s triad has formed the basis of our understanding of the pathogenesis of VTE for some time[19]
. Virchow’s model proposed that factors that increase the risk of thrombosis may be broadly divided into three categories — endothelial injury/dysfunction, stasis and altered blood constituents — and that cancer can, directly and indirectly, affect each element of the triad (see Table 1) [16],[3],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29]
.
Table 1: Virchow’s triad of coagulation and the impact of cancer upon it
| Virchow’s triad factor | Mechanism | Cancer-specific mechanism |
| Endothelial injury/dysfunction | The endothelium plays a key role in primary haemostasis through a complex interaction between platelets, the vessel wall and blood proteins. Vascular endothelium is usually anticoagulant in nature (via surface heparan sulfate), and the sub-endothelial membrane pro-coagulant in nature (owing to von Willebrand Factor [vWF]). The subsequent interaction between exposed vWF and platelets leads to clot formation. | Direct endothelial damage by: Tumour invasion of the endothelium; Secondary to chemotherapy, erythropoietins or indwelling catheters. Endothelial dysfunction: Decreased activity of vWF cleaving protein (with a resulting higher level of vWF); Increased expression of tissue factor (TF). TF is associated with secondary haemostatic process via activation of factor VII and may be up to 67% higher in cancer cells; Cancer pro-coagulant is expressed in tumour cell, which, unlike TF, can directly activate factor X. |
| Stasis | Stasis describes an alteration in blood flow; however, its role in the development of thrombosis is poorly understood. Some theories associate blood stasis, especially in the area behind venous valves (valvular sinus), with increasing hypoxia, which, in turn, leads to a downregulation of the natural, membrane-based anticoagulants. | Direct: Tumour compression of blood vessels. Indirect: Patient immobility (e.g. following hospitalisation, surgery, worsening disease). |
| Altered blood constituents | Changes in blood constituents results in direct activation of the clotting cascade. | Higher levels of certain clotting factors (e.g. V, VII, IX and X); Cancer cell release of pro-inflammatory factors, such as tumour necrosis factor alpha (TNF-α) and interlukin-1-beta (IL-1β), which can stimulate cells to become procoagulant (e.g. by increasing TF expression); Increased vascular endothelial growth factor release; Activation of leukocytes and platelets to expose procoagulant elements. |
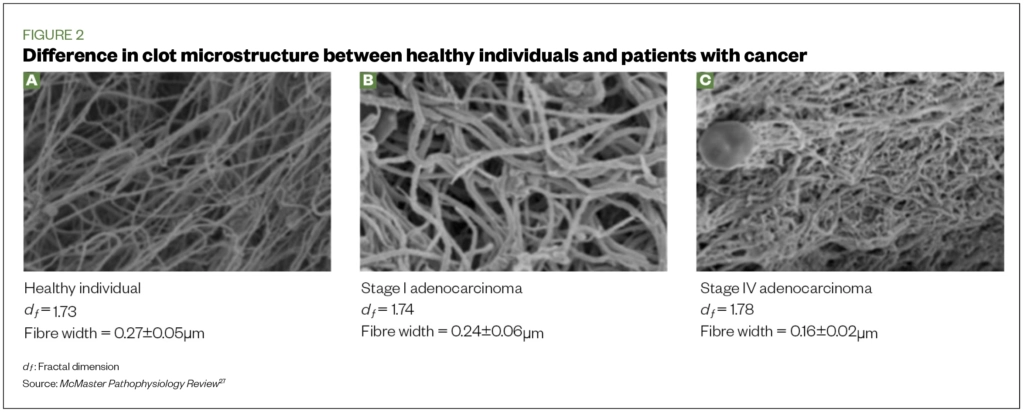
The activation of the clotting cascade differs between CAT and general thrombosis. However, research from Swansea University has shown that the clot formed in patients with advanced cancer is denser, with more fibrin branches formed within the clot microstructure (C) compared with healthy individuals (A) and those with early-stage cancer (B) (Figure 2)[30].
Pharmacists should inform those patients who want to know more about the causes of CAT that they can obtain further information from Thrombosis UK, which has a range of online articles and downloadable leaflets. Information can also be obtained from specific cancer charity websites, such as Cancer Research UK and Macmillan.
Risk factors for CAT
Risk factors for the development of CAT can be both general or specific. Common risk factors are described below and summarised in Table 2[31].
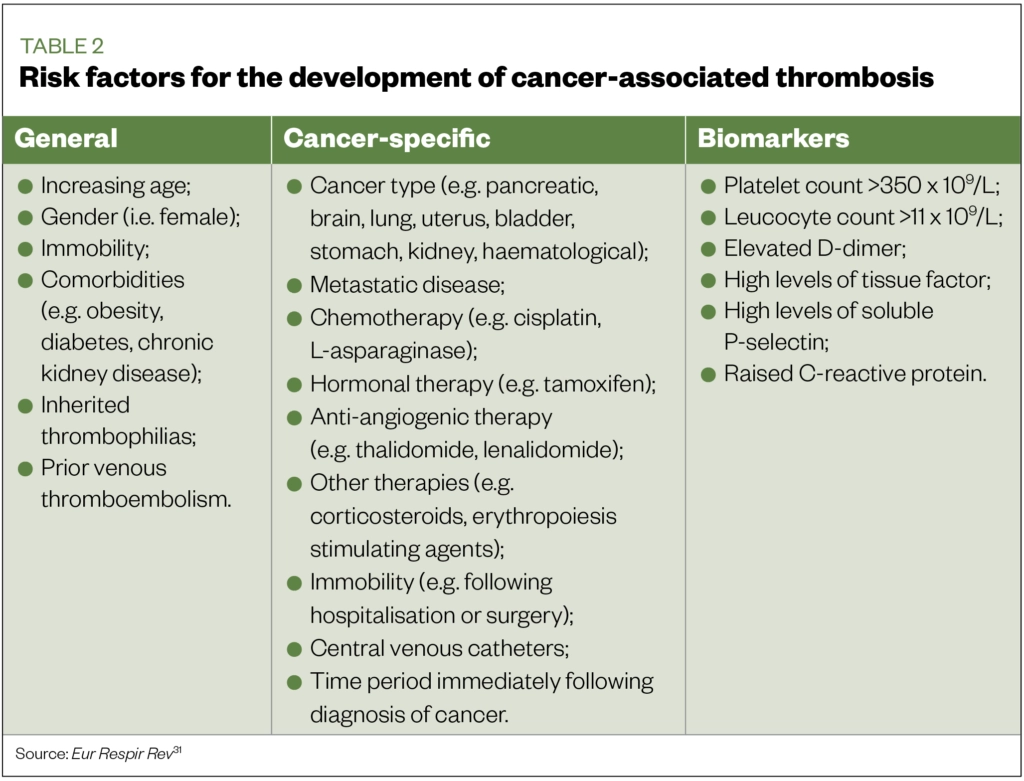
Age
Older age is a risk factor for the development of thrombosis in general, with risk increasing exponentially with advancing age[32]. This is true for CAT too, with the highest risks in patients aged 65 years and over[33].
Gender
Consensus around gender and risk has been conflicting, with some studies showing that females have a higher risk of developing CAT[33]. However, other studies have failed to show any link[34].
Immobility
Performance status (i.e. ability to carry out physical tasks) is an important prognostic marker in patients with cancer, and evidence has shown a tendency towards increasing VTE risk and worsening performance status[35],[36]. Patients should be advised to remain as active as possible throughout their cancer journey (e.g. by light exercise such as walking).
Cancer type and stage
The highest incidence of CAT are in metastatic-stage cancers of the lung, uterus, bladder, pancreas, stomach and kidney, in addition to primary brain tumours and haematological malignancies[10]. A 2011 meta-analysis identified that pancreatic cancer was associated with the highest rate of CAT[37].
Systemic anti-cancer therapy
Receiving chemotherapy is a significant risk factor — there is a 6.5-fold increased risk of a patient with cancer developing CAT while receiving chemotherapy, compared with a 4.1-fold risk in patients with cancer not receiving chemotherapy[38]. Despite a baseline annual risk for the development of CAT in patients with cancer receiving chemotherapy estimated at 11%, this may rise depending on other factors (e.g. chemotherapy type and duration of treatment)[39]. Further studies have quantified the risk based upon the time period following initiation of chemotherapy, with an overall incidence of 7.3% after 3.5 months of treatment and 13.5% at 12 months[40].
Systemic anti-cancer therapies associated with an increased risk of CAT include cisplatin, L-asparaginase, thalidomide, lenalidomide and tamoxifen[41]. A study examining the use of tamoxifen in patients with late-stage breast cancer identified a rate of CAT of up to 8% over three years[42]. The increased risk of antiangiogenic therapies, such as thalidomide, has been illustrated by a study that showed a rate of VTE of 8.9% in patients treated with the combination of a cytotoxic plus an antiangiogenic agent, compared to 3.5% in patients treated with other regimes[43]. Pharmacists should advise clinicians and patients on the individual risks of thrombosis for individual treatment strategies.
Other medications
Erythropoiesis-stimulating agents (e.g. epoetin and darbepoetin) and steroids also increase the risk of CAT. A study investigating the use of erythropoiesis-stimulating agents in patients with cancer found a rate of CAT of 14.3% in the erythropoiesis-stimulating agents’ arm, compared with 9.8% in the non-erythropoiesis-stimulating agents’ arm[44]. The use of corticosteroids has long been associated with an increased risk of thrombosis, with studies showing an increased risk of thrombosis in both cancer and non-cancer arms[45].
Central venous catheters
These are commonly used in patients with cancer, generally as a means of maintaining venous access over a prolonged time period. Data around the risk of developing CAT as a direct result of central venous catheters is difficult to interpret, often because of vague symptoms and a subsequent lack of reporting[43]. The incidence is estimated to be between 5–30%[43].
Time period following cancer diagnosis
Studies have shown the highest risk of developing CAT is in the immediate period post-diagnosis of cancer[46].
Biomarkers
Raised platelet counts (>350 x –==f33109/L), leucocyte count (>11 x 109/L), D-dimer levels, soluble P selectin, tissue factor, and C-reactive protein are markers associated with an increased risk of thrombosis in patients with cancer[47].
A study by Khorana et al found a 3.98% incidence of VTE in patients with pre-chemotherapy platelet counts of >350 x 109/L, compared with 1.25% in patients whose platelet counts were less than or equal to 350 x 109/L[48]. Likewise, another study by Khorana et al identified that patients with a leucocyte count of >11 x 109/L had a 4.5% rate of CAT, compared with 1.8% in those with a leucocyte count less than or equal to 11 x 109/L[49].
Risk of occult malignancy in unprovoked venous thromboembolism
Initial studies had suggested that in the 12-month period following a diagnosis of an unprovoked VTE, 10% of patients receive a diagnosis of cancer[50]. More recent studies have shown lower rates, with one such study showing a diagnosis rate of 3.9% within the first year of an unprovoked VTE[51]. Guidelines from the National Institute for Health and Care Excellence (NICE) advise that all non-cancer patients diagnosed with an unprovoked DVT or PE have a full history and medical examination, blood tests (full blood count, renal and liver function tests, coagulation screen) and urinalysis[52]. Previous versions of the guideline advocated the routine use of abdomino-pelvic computed tomography (CT) in patients aged over 40 years; however, the newest version only advocates further investigations in those with red flags for malignancy. The routine use of abdomino-pelvic CT is controversial, as recent evidence disputes its value[51]. The recent NICE update brings it in line with the European Society for Medical Oncology (ESMO) guidelines, which recommended against routine use of CT[53].
Assessing and reducing the risk of developing cancer-associated thrombosis
Thromboprophylaxis as primary prevention for CAT depends on factors such as the setting; bleeding and thrombotic risk factors; cost; and quality of life issues.
From a thromboprophylactic perspective, patients are generally classified as:
- Hospitalised (acute medical illness or surgery); or
- Ambulatory outpatients.
Both groups are high-risk.
Acutely unwell patients with cancer admitted to hospital
Data on the use of thromboprophylaxis for patients with cancer admitted with an acute medical illness are limited, with no specific clinical trials conducted in this area[54]. NICE does not make any specific recommendations with regards to hospitalised patients with cancer, but active cancer is one of the elements of the VTE risk assessment tool[55]. Both ESMO and American Society of Clinical Oncology (ASCO) guidelines support the use of thromboprophylaxis in patients with cancer admitted to hospital with an acute medical illness[53],[56]. Therefore, pharmacists should ensure that all admitted patients with cancer are assessed for their risk of hospital-acquired thrombosis, and ensure that the correct thromboprophylactic anticoagulation is prescribed.
Surgical patients with cancer
Patients with cancer undergoing surgery are known to have a higher risk of post-operative DVT than non-cancer patients, with VTE rates potentially two-fold higher in patients with cancer who underwent gastrointestinal surgery compared with non-cancer patients[57]. Both the NICE and ESMO guidelines recommend extending thromboprophylaxis to up to four weeks in patients with cancer who have undergone major abdominal surgery for cancer[53],[55]. This recommendation is based upon two studies that showed that the risk of VTE in patients with cancer undergoing major abdominal or pelvic surgery was reduced by up to 60% if thromboprophylaxis with a low molecular weight heparin (LMWH) was extended up to 30 days[58],[59]. Pharmacists should ensure that patients are prescribed appropriate extended thromboprophylaxis on discharge, and ensure that there are safe methods of the patient having their medication administered, either by patient education for self-administration or facilitation of district nurse visits.
Ambulatory patients with cancer receiving chemotherapy
NICE recommends that thromboprophylaxis is not generally considered in ambulatory patients with cancer who are receiving cancer-modifying treatments, unless they are at risk of VTE because of something other than cancer[55]. However, there are two exceptions to this rule:
- Patients with myeloma receiving chemotherapy with thalidomide, pomalidomide or lenalidomide with steroids. For these patients, NICE recommends considering pharmacological thromboprophylaxis with either aspirin (75mg or 150mg) or LMWH[55]. The British Society for Haematology (BSH) expanded this further by recommending patients
with a low risk use aspirin and those at high risk use LMWH[60]. Clinical trial data demonstrated the efficacy and cost-effectiveness of aspirin in this patient group[61],[62],[63]. - NICE recommends specifically that pharmacological VTE prophylaxis, with LMWH, be considered for patients with pancreatic cancer who are receiving chemotherapy[55]. This recommendation is based upon the high rate of CAT in this population and the benefit of thromboprophylaxis. The CONKO study showed that LMWH (i.e. enoxaparin 1mg/kg once daily) use in patients with pancreatic cancer undergoing chemotherapy produced a 6.4% overall cumulative incidence rate of symptomatic CAT, compared with a rate of 15.1% in the comparator arm (hazard ratio 0.40; 95% confidence interval [CI]: 0.19–0.83; P =0.01)[64]. Subsequently, the FRAGEM study evaluated the impact of dalteparin (200 units/kg once per day for four weeks, followed by 150 units/kg once per day for eight weeks) being added to gemcitabine for the treatment of pancreatic cancer. Similar findings were observed to CONKO, with the rate of symptomatic VTE reduced from 23% to 3.4% (risk ratio 0.145; 95% CI: 0.035–0.612) with a similar rate of bleeding[65].
There is little evidence to support the routine use of thromboprophylaxis in ambulatory patients with other types of cancers. A Cochrane review of nine randomised controlled trials (eight LMWH and one warfarin, n=3538) compared patients receiving thromboprophylaxis with control groups, and found a reduction in VTE risk (relative risk [RR] 0·66 [0·41–0·93]) without a significant increased risk of bleeding (RR 1·57 [0·69–3·6]). This equates to 60 patients requiring treatment to prevent one episode of thrombosis, suggesting that thromboprophylaxis should not be used routinely in outpatients with cancer, but should be considered in individuals at very high thrombotic risk[66].
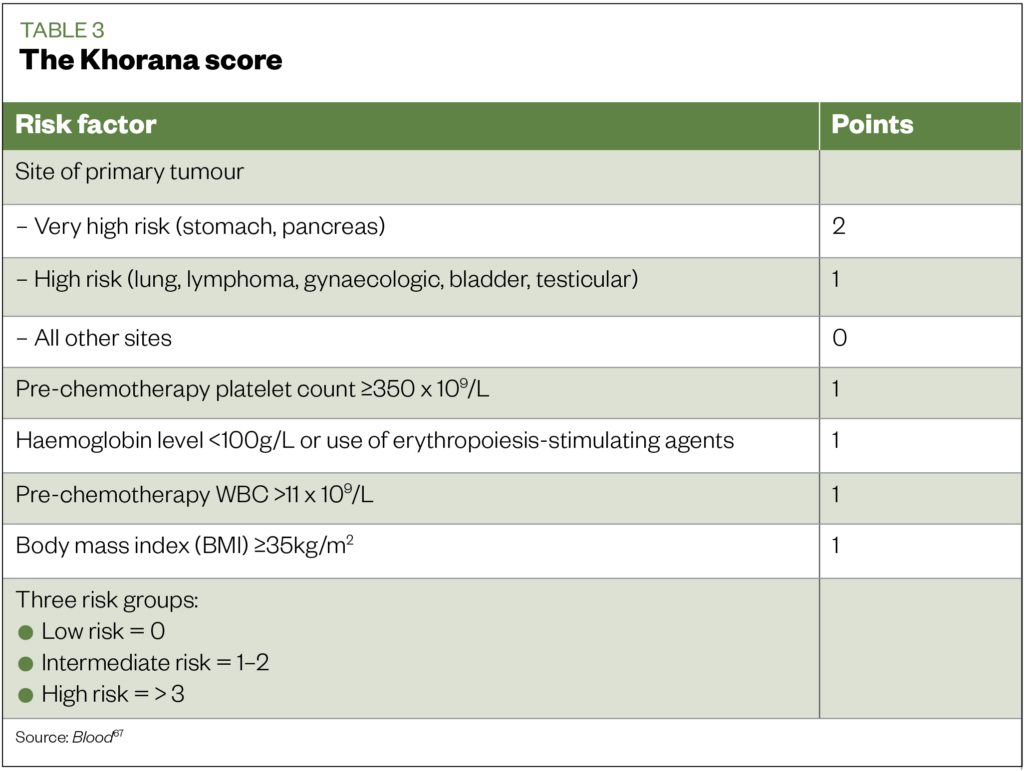
Identification of these high-risk patients can be aided by the use of risk assessment tools, such as the Khorana score (see Table 3)[67]. This validated tool estimates VTE risk in ambulatory patients with cancer receiving chemotherapy. In the PROTECT study, which compared the use of the LMWH nadroparin with placebo in ambulatory chemotherapy patients, sub-group analysis identified the greatest benefit of therapy to be in high-risk patients, i.e. those with a Khorana score ≥3 (numbers needed to treat [NNT] 15 for high-risk patients and 77 for low to intermediate patients)[68].
Although previous guidance has only advocated the use of LMWH in preventing CAT[69], the role of direct oral anticoagulants (DOACs) has also been assessed recently, with the publication of the AVERT and CASSINI studies. These studies examined the use of thromboprophylaxis in high-risk (Khorana score ≥2) ambulatory patients with cancer starting chemotherapy using either apixaban 2.5mg twice daily and rivaroxaban 10mg daily for
180 days respectively[70],[71].
Use of apixaban in the AVERT study was associated with a significantly lower incidence of CAT than placebo, but with a higher incidence of major bleeding episodes[70]. Use of rivaroxaban in the CASSINI study had a lower incidence of CAT than placebo in the per-protocol analysis, but not in the primary intention-to-treat analysis, with no significant difference in major bleedings[71].
However, it should be noted that Doppler ultrasound screening was undertaken prior to randomisation in the CASSINI study, and the presence of DVT resulted in the patient being excluded from the study. As this was not undertaken as part of the AVERT study, this may explain the difference in the efficacy outcomes findings for the studies (i.e. there were less VTE-threatened patients in the CASSINI study). Prior to randomisation, DVT was identified in 4.5% (n=49) of patients in the CASSINI study, highlighting the small, yet appreciable risk of asymptomatic DVT in the patient with cancer.
A combined analysis of AVERT and CASSINI studies found a small absolute reduction (2.5% or NNT 24) in the risk of CAT, with a small increase in the risk of major bleeding (NNH 77)[72]. The on-treatment analysis identifies an NNT of 26 for the CASSINI trial and an NNT of 16 in AVERT[70],[71]. It is also worth noting the high adherence rates for the on-treatment arms of both studies (98.4% in CASSINI and 83.6% in AVERT)[70],[71]. Adherence with therapy is naturally important to ensure efficacy of therapies, as highlighted previously in the article.
In 2019, ASCO recommend that high-risk outpatients with cancer (Khorana score of 2 or higher prior to starting a new systemic chemotherapy regimen) may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, provided there are no significant risk factors for bleeding and no drug interactions. Consideration of such therapy should be accompanied by a discussion with the patient about the relative benefits and harms, drug cost and duration of prophylaxis in this setting[56].
Prevention of catheter-related thrombosis
A Cochrane review evaluated the efficacy of oral and parenteral anticoagulants in the prevention of central venous catheter related thrombosis and found no associated reduction in risk with either warfarin or prophylactic dose LMWH[73]. Therefore, the routine use of anticoagulants at prophylactic or therapeutic dose to prevent catheter‐related thrombosis in patients with cancer is not recommended[61].
Increasing patient awareness
Cancer Research UK consent forms for systemic anti-cancer therapy (SACT) now include a specific section to ensure that the risks of developing CAT are discussed with patients, stating: “Cancer can increase your risk of developing a blood clot (thrombosis), and having treatment with anti-cancer medicines may increase this risk further. A blood clot may cause pain, redness and swelling in a leg, or breathlessness and chest pain — you must tell your doctor straight away if you have any of these symptoms”[74].
Diagnosis
No specific guidelines for the diagnoses of CAT exist and, instead, diagnosis is as per current general VTE guidelines (e.g. NICE)[52]. Active cancer is an element of the two-level Wells DVT and PE scores, meaning that patients will automatically score a point purely on their cancer status[49].
However, there is confusion around the use of D-dimer levels (protein fragments made when a blood clot dissolves) in patients with cancer. D-dimer levels are generally elevated in patients with cancer, hence their value is debatable[75],[76]. A study exploring the use of D-dimer in PE patients found that the D-dimer was only found to be negative in one in ten patients with cancer at the usual cut-off value (i.e. <500ug/l), hence research is currently focused on exploring the use of higher-level D-dimer cut offs[77]. For confirmation of a diagnosis of CAT, NICE recommends an ultrasound Doppler scan for DVT, and a CT pulmonary angiogram for PE[52].
Management
Anticoagulant therapies are the cornerstone of treatment of patients with CAT. The aims of acute treatment (within the first six months) of CAT are to prevent fatal PE, recurrent CAT, clot extension and long-term complications (e.g. post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension).
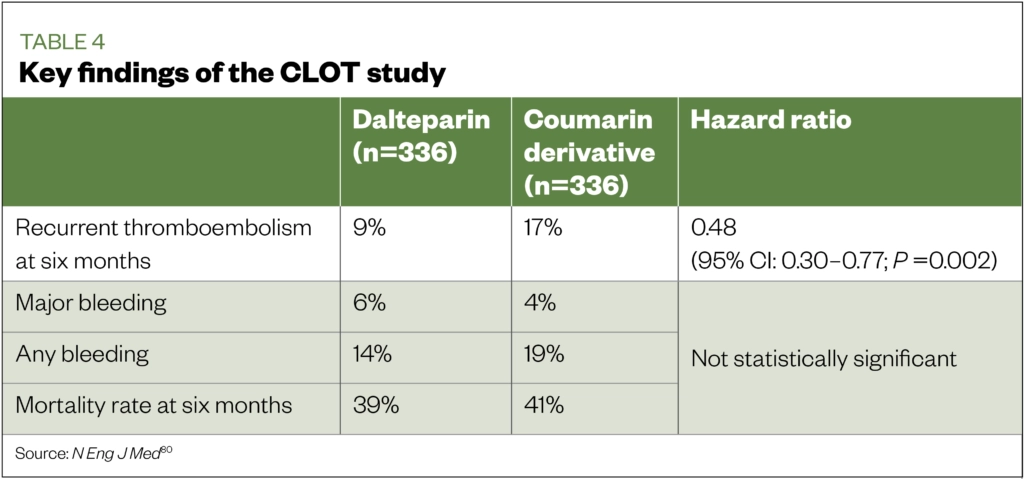
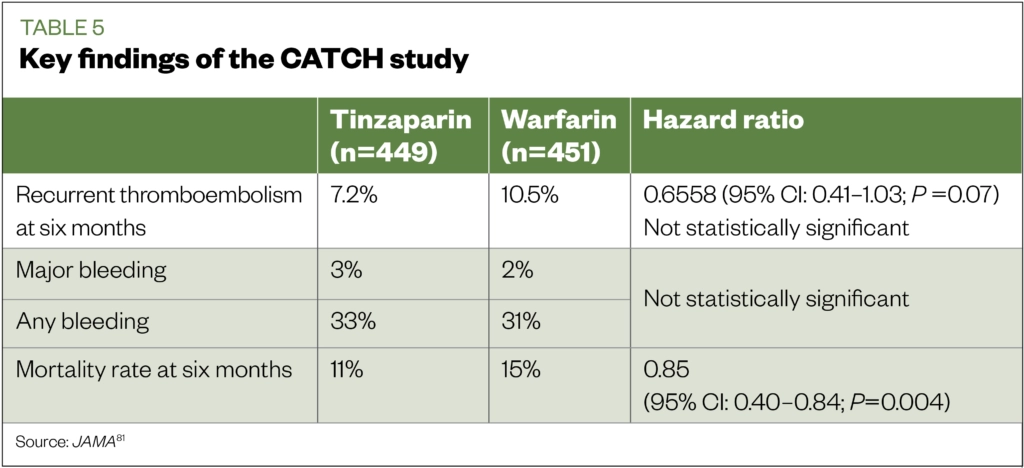
The primary treatment option is LMWHs. Dalteparin and tinzaparin are currently the only anticoagulants licensed for treatment of CAT[78],[79]. The evidence supporting the use of LMWHs in CAT comes from the CLOT and the CATCH studies[80],[81].
In the CLOT study (n=676), dalteparin significantly reduced the risk of CAT compared with coumarin derivatives (see Table 4) and no statistically significant difference in bleeding rates were observed[80].
In the CATCH (n=900) study, tinzaparin was found to be non-inferior to warfarin in reducing the risk of recurrent thrombosis compared with warfarin (see Table 5)[81]. Although the CATCH study did not show statistical significance in the primary outcomes of recurrent VTE, as observed in the CLOT study, this is likely owing to differences in the study design, such as higher number of active cancer patients in the CLOT study compared with the CATCH study (72% vs 53%); previous history of VTE (11% vs 6%); metastatic disease (67% vs 55%); mortality rates at six months (39% vs 33%); and poorer performance scores in the CLOT study[80],[81].
The CLOT study showed treatment of CAT with six months of LMWH had a significantly lower recurrence rate than conventional treatment with international normalized ratio‐adjusted warfarin. This forms the evidence base on which national and international organisations have based their recommendations to use LMWHs for six months as first-line treatment option[53],[56],[60].
However, there is growing evidence supporting the use of DOACs in the treatment of CAT with edoxaban (Hokusai-VTE Cancer study), rivaroxaban (SELECT-D) and apixaban (ADAM VTE and Caravaggio)[82-85]. The first two studies (Hokusai-VTE Cancer and SELECT-D) showed a general trend towards better efficacy in favour of the DOACs, but worse safety outcomes (especially in patients with gastrointestinal and urothelial cancers)[82],[83].
Caravaggio showed apixaban to be non-inferior to dalteparin in terms of efficacy, and showed similar bleeding risk. However, the lack of homogeneity between studies should be noted; for example, patients with primary brain cancers or intracerebellar metastases were excluded from Caravaggio, but not Hokusai or SELECT-D[82],[83],[85]. It should also be noted that the clinical studies in CAT are with apixaban, edoxaban and rivaroxaban (all of which are factor Xa inhibitors), and little information is available for dabigatran (a thrombin inhibitor). A summary of the findings of the key DOAC studies can be found in Table 6[82-85].
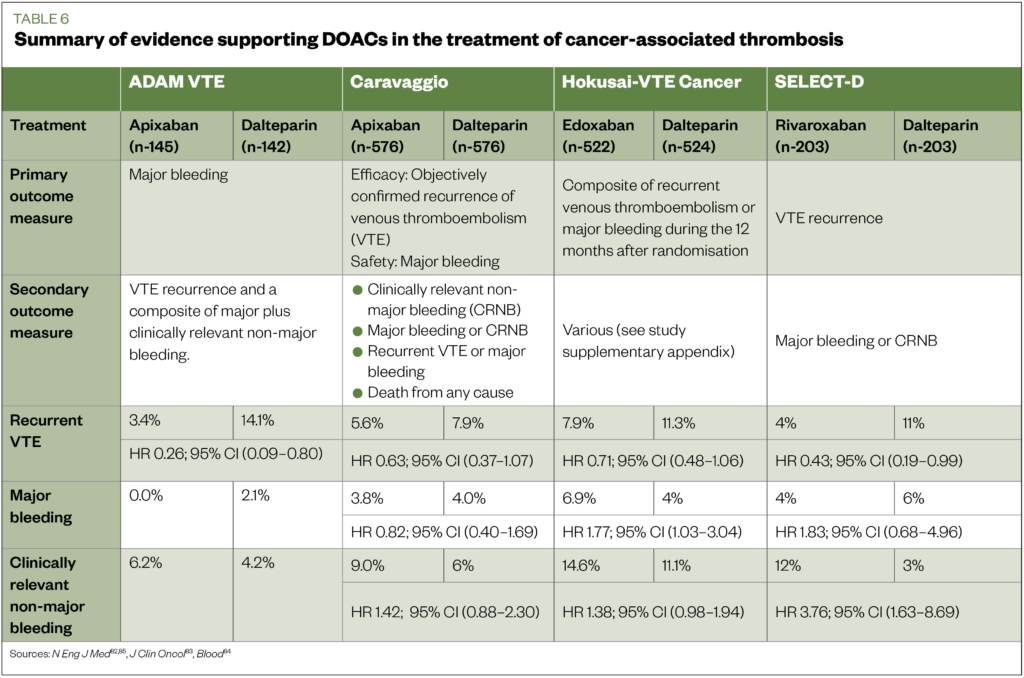
Based upon the above studies, the International Society on Thrombosis and Haemostasis (ISTH) in 2018, and ASCO in 2019, published guidance supporting the use of LMWH, edoxaban or rivaroxaban as treatment options in CAT patients for at least six months[56],[86]. ASCO prefers LMWH or DOACS (e.g. edoxaban and rivaroxaban) because of improved efficacy over vitamin K antagonists (VKAs). However, it stated that although VKAs are inferior, they may be used if LMWH or DOACs are not accessible[56]. The newest NICE guidance on the diagnosis and management of VTE also advocate DOACS as first-line treatments[52]. It should be noted that, at the time of the publication of most of the above recommendations, only edoxaban and rivaroxaban had cancer-specific studies[82],[83]. However, with the publication of the Caravaggio study, apixaban can be considered to have evidence to support its use in cancer[85].
Considering the previously mentioned lack of heterogeneity between DOAC CAT studies, it is difficult to advocate one DOAC over the other for the cancer population as a whole. Instead, clinicians will need to consider the above data in relation to individual patient factors (e.g. cancer type, interaction, clinical characteristics such as renal function). It is necessary to also consider that each anticoagulant therapy has, based on the authors’ opinion and experience, practical advantages and disadvantages when used in patients with cancer (see Table 7). In addition, differences in the dosing regimens and routes of administration exist (see Table 8)[76-80]. These practical differences may exist even within the DOAC group; for example, edoxaban requires a lead-in with LMWH for at least five days, whereas apixaban and rivaroxaban do not[82],[83],[85].
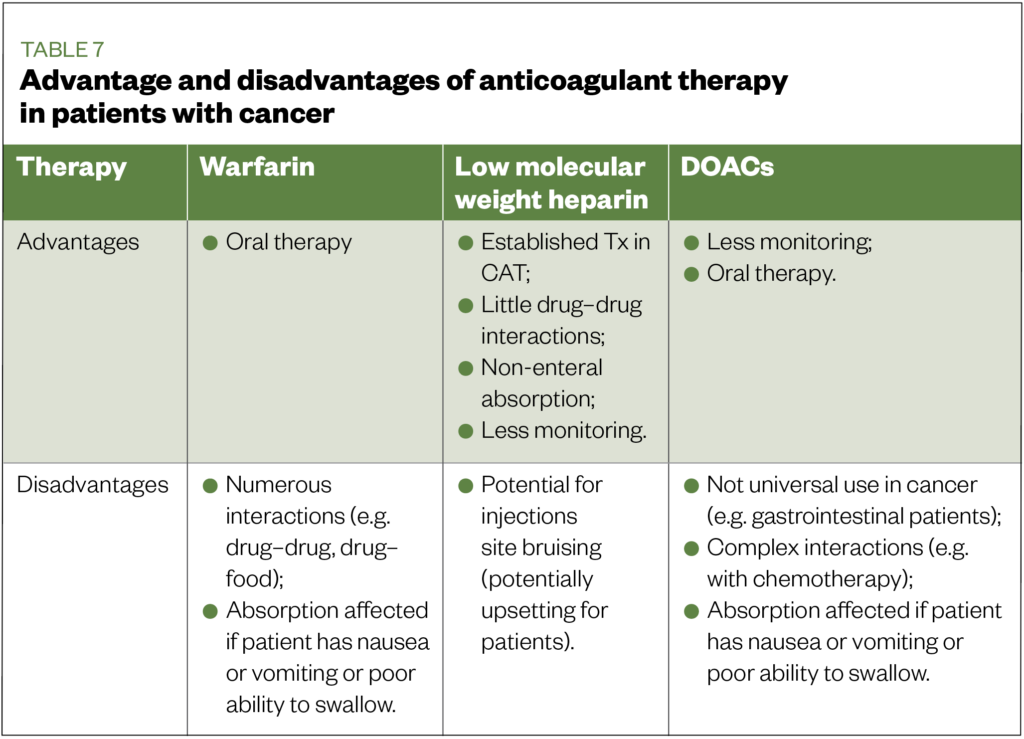
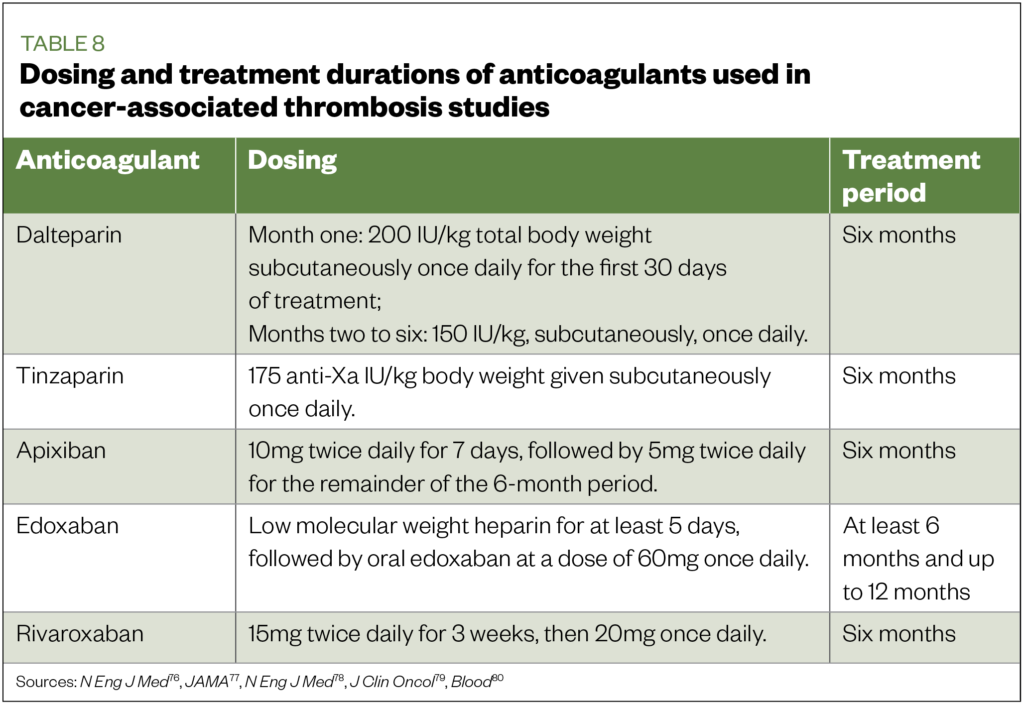
One of the key recommendations was that clinicians should individualise treatment, adapting a shared-care approach. Pharmacists can play a key role in the development of individualised care plans for the management of CAT patients, via their knowledge of the evidence for these therapies, and understanding of the practical differences that exist for each.
Incidental VTE should also be managed; however, there is a lack of evidence and consensus on how to manage these types of thrombi, leading to some confusion around what is the best method. Based on data showing a similar mortality rate being observed for both symptomatic and incidental PEs, guidelines recommend that incidental VTE is managed in the same way as symptomatic VTE[69]. This is except for incidental sub-segmental PEs and visceral vein thrombi, which are managed on a case-by-case basis, depending on thrombotic and bleeding risks.
Management beyond six months
Evidence and clear consensus on how to manage anticoagulation beyond the six-month point is currently lacking, but the consensus is:
- Continue anticoagulation if patient still has active cancer (especially if metastatic disease and/or receiving SACT);
- Decisions should be made on an individual patient-to-patient basis, taking into consideration factors such as risk of re-occurrence, bleeding, interactions, patient’s preference for oral or parenteral drug administration, and patient wishes.
Observational LMWH studies have been undertaken for dalteparin (the DALTECAN study), and tinzaparin (the TiCAT study) for patients requiring extended anticoagulation up to 12 months[87],[88]. Both studies showed no increase in bleeding rates or increases in re-occurrence rates with longer-term use. The Hokusai-VTE Cancer study (edoxaban) evaluated patient outcomes up to 12 months post-diagnosis[82]. However, as seen in Table 6, a significant increase in major bleeding, especially in patients with gastrointestinal or urological cancers, was observed. Most guidelines recommend continuing with LMWH therapy if anticoagulant therapy is to continue beyond the six‐month point; however, oral options can be considered if patients are unable to tolerate LMWH[52],[53],[56].
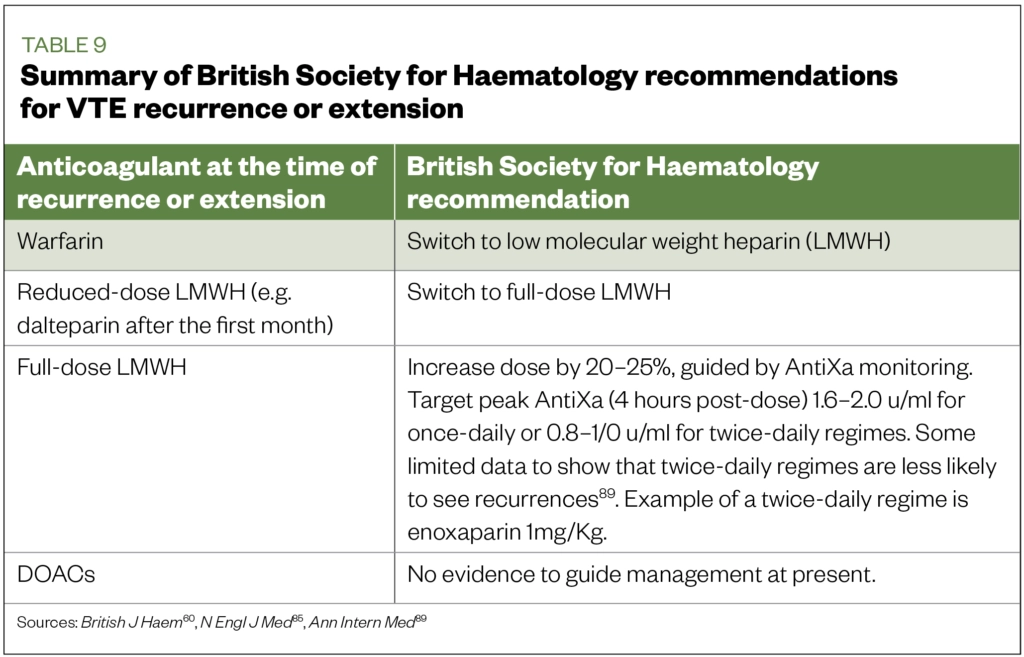
Recurrence or clot extension on anticoagulant therapy
The CLOT study reported a VTE/CAT recurrence rate of between 6% and 9%[81]. In cases of recurrence or extension, BSH guidelines recommendations are summarised in Table 9[60],[89].
Financial and conflict of interest disclosure
Kieron Power has an honoraria from Leo Pharma and Pfizer BMS. The authors have no other relevant affiliations of financial involvement with any other organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of
this manuscript.
References
[1] All Party Parliamentary Group. Venous thromboembolism (VTE) in cancer patients: cancer, chemotherapy and clots. 2016. Available at: https://www.thrombosisuk.org/downloads/apptg-vte-in-cancer-patients-report-2016.pdf (accessed September 2020)
[2] Noble S & Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010;102:S2–S9. doi: 10.1038/sj.bjc.6605599
[3] White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66
[4] Puurunen MK, Gona PN, Larson MG et al. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thrombo Res 2016;145:27–33. doi: 10.1016/j.thromres.2016.06.033
[5] Sørensen HT, Mellemkjær L, Olsen JH & Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504
[6] Blom JW, Doggen CJM, Osanto S et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–722. doi: 10.1001/jama.293.6.715
[7] Khorana AA, Francis CW, Culakova E et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thrombo Haemost 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x
[8] Bozas G, Jeffery N, Ramanujam-Venkatachala D et al. Prognostic assessment for patients with cancer and incidental pulmonary embolism. Thrombosis J 2018;16,8. doi: 10.1186/s12959-017-0157-x
[9] Cohen AT, Katholing A, Rietbrock S et al. Epidemiology of the first and recurrent venous thromboembolism in patients with active cancer: a population-based cohort study. Thromb Haemost 2017;117:57–65. doi: 10.1160/TH15-08-0686
[10] Chew HK, Wun T, Harvey D et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166(4):458–464. doi: 10.1001/.458
[11] Fahrni J, Husmann M, Gretener SB & Keo HH. Assessing the risk of recurrent venous thromboembolism: a practical approach. Vasc Health Risk Mang 2015;11:451–459. doi: 10.2147/VHRM.S83718
[12] Mandala M, Falanga A & Rolia F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(S6):vi85–vi92. doi: 10.1093/annonc/mdr392
[13] Noble S, Prout H & Nelson A. Patients’ experiences of living with cancer-associated thrombosis: the PELICAN study. Patient Prefer Adherence 2015;9:337–345. doi: 10.2147/PPA.S79373
[14] Noble S, Matzdorff A, Maraveyas A et al. Assessing patients’ anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica 2015;100:1486–1492. doi: 10.3324/haematol.2015.127126
[15] Noble S, Pease N, Sui J et al. Impact of a dedicated cancer-associated thrombosis service on clinical outcomes: a mixed-methods evaluation of a clinical improvement exercise. BMJ Open 2016;6:e013321. doi: 10.1136/bmjopen-2016-013321
[16] Bouillard JB, Bouillaud S. De l’Obliteration des veines et de son influence sur la formation des hydropisies partielles: consideration sur la hydropisies passive et general. Arch Gen Med 1823;1:188–204. Available at: https://www.scienceopen.com/document?vid=e001c8cd-a3cf-4d55-95c7-952530ea1f95 (accessed September 2020)
[17] Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost 2003;1:2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x
[18] Trousseau A. Phlegmasia alba dolens. In: Clinique Médicale de l’Hôtel-Dieu de Paris. Paris: J. B. Baillière et Fils; 1865. Available at: https://archive.org/details/cliniquemdicaled01trou (accessed September 2020)
[19] Kumar DR, Hanlin E, Glurich I & Mazza JJ. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res 2010;8(3–4):168–172. doi: 10.3121/cmr.2009.866
[20] Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol 1974;27(7):517–528. doi: 10.1136/jcp.27.7.517
[21] Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth 2014;58(5):515–523. doi: 10.4103/0019-5049.144643
[22] Heemskerk JW, Bevers EM & Lindhout T. Platelet activation and blood coagulation. Thromb Haemost 2002;88:186–193. PMID: 12195687
[23] Smith SA, Travers RJ & Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol 2015;50(4):326–336. doi: 10.3109/10409238.2015.1050550
[24] Lyman GH & Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol 2009;27(29):4821–4826. doi: 10.1200/JCO.2009.22.3032
[25] Oleksowicz L, Bhagwati N & DeLeon-Fernandez M. Deficient activity of von Willebrand’s factor-cleaving protease in patients with disseminated malignancies. Cancer Res 1999;59:2244–2250. Available at: https://cancerres.aacrjournals.org/content/59/9/2244 (accessed September 2020)
[26] Kakkar AK, DeRuvo N, Chinswangwatanakul V & Tebbutt S. Extrinsic-pathway activation in cancer with high factor VIIa and tissue factor. Lancet 1995;346:1004–1005. doi: 10.1016/S0140-6736(95)91690-3
[27] Wong E & Chaudhry S. Venous thromboembolism (VTE). McMaster Pathophysiology Review. Available at: http://www.pathophys.org/vte (accessed September 2020)
[28] Falanga, Marchetti M & Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013;11(2):223–233. doi: 10.1111/jth.12075
[29] Hamer JD, Malone PC, Silver IA. TheP O2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg 1981;68:166–170. doi: 10.1002/bjs.1800680308
[30] Davies NA, Harrison NK, Morris RHK et al. Fractal dimension (df) as a new structural biomarker of clot microstructure in different stages of lung cancer. Thromb Haemost 2015;114(06):1251–1259. doi: 10.1160/TH15-04-0357
[31] Fernandes CJ, Morinage LTK, Alves JL et al. Cancer-associated thrombosi: the when, how and why. Eur Respir Rev 2019;28:180119. doi: 10.1183/16000617.0119-2018
[32] Silverstein MD, Heit JA, Mohr DN et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–593. doi: 10.1001/archinte.158.6.585
[33] Khorana AA, Francis CW, Culakova E et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;110(10):2339–2346. doi: 10.1002/cncr.23062
[34] Chew HK, Wun T, Harvey D et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458–464. doi: 10.1001/archinte.166.4.458
[35] Khorana AA, Francis CW, Culakova E & Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822–2829. doi: 10.1002/cncr.21496
[36] Al Diab AI. Cancer-related venous thromboembolism: insight into underestimated risk factors. Hematol Oncol Stem Cell Ther 2010;3(4):191–195. doi: 10.5144/1658-3876.2010.191
[37] Horsted F, West J & Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med 2012;9:e1001275. doi: 10.1371/journal.pmed.1001275
[38] Heit JA, Silverstein MD, Mohr DN et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160,809–815. doi: 10.1001/archinte.160.6.809
[39] Haddad TC & Greeno EW. Chemotherapy-induced thrombosis. Thromb Res 2006;118(5):555–568. doi: 10.1016/j.thromres.2005.10.015
[40] Lyman G, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology practice guideline update. J Clin Oncol 2013; 31(17):2189–2204. PMID: 31381464
[41] Abdol Razak NB, Jones G, Bhandari M et al. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers 2018;10(10):380. doi: 10.3390/cancers10100380
[42] Deitcher SR & Gomes MPV. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review. Cancer 2004;101(3):439–449. doi: 10.1002/cncr.20347
[43] Mandalà M, Grosso F, Vitalini C et al. Venous thromboembolism is a relevant and underestimated adverse event in cancer patients treated in phase I studies. Br J Cancer 2012;107(4):612–616. doi: 10.1038/bjc.2012.325
[44] Hershman D, Buono DL, Malin J et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst 2009;101(23):1633–1641. doi: 10.1093/jnci/djp387
[45] Johannesdottir SA, Horváth-Puhó E, Dekkers OM et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173(9):743–752. doi: 10.1001/jamainternmed.2013.122
[46] Khorana AA & Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 2009;27:4839–4847. doi: 10.1200/JCO.2009.22.3271
[47] Elyamany G, Alzahrani AM & Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol 2014;8:129–137. doi: 10.4137/CMO.S18991
[48] Khorana AA, Francis CW, Culakova E & Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822–2829. doi: 10.1002/cncr.21496
[49] Connolly GC, Khorana AA, Kuderer NM et al. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res 2010;126(2):113–118. doi: 10.1016/j.thromres.2010.05.012
[50] Carrier M, Le Gal G, Wells PS et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008;149:323–333. doi: 10.7326/0003-4819-149-5-200809020-00007
[51] Carrier M, Lazo-Langer A, Shivakumar S et al. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med 2015;373(8):697–704. doi: 10.1056/NEJMoa1506623
[52] National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NICE guideline [NG158]. 2020. Available at: https://www.nice.org.uk/guidance/ng158 (accessed September 2020)
[53] Mandala M, Falanga A & Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(Suppl 6):vi85–vi92. doi: 10.1093/annonc/mdr392
[54] Carrier M, Khorana AA, Moretto P et al. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med 2014;127(1):82–86 e81. doi: 10.1016/j.amjmed.2013.09.015
[55] National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. NICE guideline [NG89]. 2019. Available at: https://www.nice.org.uk/guidance/ng89 (accessed September 2020)
[56] Lyman GH, Bohlke K, Khorana AA et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;33(6):654–656. doi: 10.1200/JCO.2014.59.7351
[57] Kakkar AK & Williamson RCN. Prevention of venous thromboembolism in cancer patients. Semin Thromb Hemost 1999;25:239–243. doi: 10.1055/s-2007-994925
[58] Bergqvist D, Agnelli G, Cohen AT et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–980. doi: 10.1056/NEJMoa012385
[59] Rasmussen MS, Jorgensen LN, Wille-Jørgensen P et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x
[60] Watson HG, Keeling DM, Laffan M et al. British Committee for Standards in Haematology. Guideline on aspects of cancer-related venous thrombosis. British J Haem 2015;170(5):640–648. doi: 10.1111/bjh.13556
[61] Palumbo A, Cavo M, Bringhen S et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 2011;29(8):986–993. doi: 10.1200/JCO.2010.31.6844
[62] Larocca A, Cavallo F, Bringhen S et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119(4):933–939. doi: 10.1182/blood-2011-03-344333
[63] Chalayer E, Bourmaud A, Tinquat F et al. Cost-effectiveness analysis of low-molecular-weight heparin versus aspirin thromboprophylaxis in patients newly diagnosed with multiple myeloma. Thromb Res 2016;145:119–125. doi: 10.1016/j.thromres.2016.08.008
[64] Pelzer U, Opitz B, Deutschinoff G et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 2015;33(18):2028–2034. doi: 10.1200/JCO.2014.55.1481
[65] Maraveyas A, Waters J, Roy R et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48(9):1283–1292. doi: 10.1016/j.ejca.2011.10.017
[66] Di Nisio M, Porreca E, Ferrante N et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2012;29,CD008500. doi: 10.1002/14651858.CD008500.pub2
[67] Khorana AA, Kuderer NM, Culakova E et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902. doi: 10.1182/blood-2007-10-116327
[68] Agnelli G, Gussoni G, Bianchini C et al. A randomized double-blind placebo-controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: the PROTECHT study. Blood 2018;112(11)6. Available at: http://www.bloodjournal.org/content/112/11/6 (accessed September 2020)
[69] Khorana AA, Carrier M, Garcia DA & Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016;41(1):81–91. doi: 10.1007/s11239-015-1313-4
[70] Carrier M, Abou-Nassar K, Mallick R et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380(8):711–719. doi: 10.1056/NEJMoa1814468
[71] Khorana AA, Soff GA, Kakkar AK et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;380(8):720–728. doi: 10.1056/NEJMoa1814630
[72] Agnelli G. Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med 2019;380:781–783. doi: 10.1056/NEJMe1816060
[73] Akl EA, Vasireddi SR, Gunukula S et al. Anticoagulation for patients with cancer and central venous catheters. Cochrane Database Syst Rev 2011;13:CD006468. doi: 10.1002/14651858.CD006468.pub4
[74] Cancer Research UK. SACT consent forms. Cancer Research UK. Available at: https://www.cancerresearchuk.org/sites/default/files/generic_form_pdf_v4.pdf (accessed September 2020)
[75] Lee AY, Julian JA, Levine MN et al. Clinical utility of a rapid whole-blood D-dimer assay in patients with cancer who present with suspected acute deep venous thrombosis. Ann Intern Med 1999;131:417–423. doi: 10.7326/0003-4819-131-6-199909210-00004
[76] Carrier M, Lee AY, Bates SM et al. Accuracy and usefulness of a clinical prediction rule and D-dimer testing in excluding deep vein thrombosis in cancer patients. Thromb Res 2008;123:177–183. doi: 10.1016/j.thromres.2008.05.002
[77] Righini M, Le Gal G, De lucia S et al. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb Haemost 2006;95:715–719. doi: 10.1160/TH05-12-0791
[78] Electronic medicines compendium. Fragmin 18,000 IU/0.72ml solution for injection. 2020. Available at: https://www.medicines.org.uk/emc/product/4243/smpc (accessed September 2020)
[79] Electronic medicines compendium. innohep syringe 20,000 IU/ml solution for injection in pre-filled syringe. 2020. Available at: https://www.medicines.org.uk/emc/product/3631/smpc (accessed September 2020)
[80] Lee AY, Levine MN, Baker RI et al. Low molecular weight heparin versus a coumarin for prevention of recurrent venous thromboembolism in patients with cancer. N Eng J Med 2003;349:146–153. doi: 10.1056/NEJMoa025313
[81] Lee AY, Kamphuisen PW, Meyer G et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomised clinical trial. JAMA 2015;314:677–386. doi: 10.1001/jama.2015.9243
[82] Raskob GE, Van Es N, Verhamme P et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615–624. doi: 10.1056/NEJMoa1711948
[83] Young AM, Marshall A, Thirlwall J et al. Comparison of an oral factor Xa Inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36(20):2017–2023. doi: 10.1200/JCO.2018.78.8034
[84] McBane RD, Wysokinski WE, Le-Rademacher J et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood 2018;132:421. doi: 10.1182/blood-2018-99-118808
[85] Agnelli G, Becattini C, Meyer G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020;382:1599–1607. doi: 10.1056/NEJMoa1915103
[86] Khorana AA, Noble S, Lee AYY et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891–1894. doi: 10.1111/jth.14219
[87] Francis CW, Kessler CM, Goldhaber SZ et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN study. J Thromb Haemost 2015;13:1028–1035. doi: 10.1111/jth.12923
[88] Jaraâ€Palomares L, Solier-Lopez A, Elias-Hernandez T et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thromb Res 2017;157:90–96. doi: 10.1016/j.thromres.2017.07.004
[89] Merli G, Spiro TE, Olsson CG et al. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med 2001;134(3);191–202. doi: 10.7326/0003-4819-134-3-200102060-00009
You might also be interested in…

Participation from pharmacy in shaping future royal college strategy ‘critical’, RPS conference delegates told

Tips for effective lipid management consultations for secondary prevention of cardiovascular disease
