
Shutterstock
After reading this article, you should be able to:
- Understand the rationale for tapering antidepressants gradually, in a hyperbolic pattern and a rate titrated to the individual;
- Counsel a patient on how to undertake a careful, hyperbolic taper of antidepressants;
- Help a patient use different formulations of medication to facilitate this process.
It is now recognised that withdrawal effects from antidepressants are more common, and can be more severe and long-lasting than previously understood. This prompted a report on the topic by Public Health England in 2020, a change in position from the Royal College of Psychiatrists, and an update to National Institute of Health and Care Excellence (NICE) guidance[1–3]. Around half of patients will experience withdrawal effects from antidepressants, with NICE guidelines indicating that withdrawal effects can be severe and can last “several months”[4,5]. For some patients, antidepressant withdrawal effects can last for years and can be debilitating, leading to job loss, relationship breakdown and even (although rarely) suicide[6,7]. The extent of these effects has been poorly recognised by clinicians[8,9]. Both NICE and the Royal College of Psychiatrists recommend that patients are informed of the potential for severe and/or long-lasting withdrawal effects when considering starting an antidepressant[2,10].
The words ‘dependence’ and ‘addiction’ have come to be used interchangeably in common parlance, following a decision by the DSM-III-R committee to use the word ‘dependence’ in place of ‘addiction’ because it was thought less pejorative[11]. In pharmacology, however, physical dependence refers to “physiological adaptation that occurs when medications acting on the central nervous system are ingested”[12]. The appearance of a withdrawal syndrome is the “only actual evidence of a physical dependence”[13]. Antidepressants are not addictive (i.e. they do not produce craving, compulsion, etc.) but do engender physical dependence. It is now generally recognised that ‘withdrawal symptoms’, not the industry euphemism ‘discontinuation symptoms’, is the correct scientific terminology[2,14].
NHS England has released a commissioning framework that includes a call for the establishment of services to help people safely stop antidepressants[15]. One of the ‘national medicines optimisation opportunities’ from NHS England relates to addressing inappropriate antidepressant prescribing[16]. Several integrated care boards have recognised that community and primary care pharmacists are well positioned to advise and support patients on how to safely taper and stop antidepressants[17].
Physical dependence
Withdrawal effects from antidepressants occur because, over time, the brain adapts to the presence of antidepressants in a manner that opposes their effects (the drive to homeostasis)[18]. Down-regulation of serotonin receptors is evident in nuclear imaging of patients after several weeks of antidepressant treatment, and has been detected up to four years after antidepressants have been ceased[18,19]. Other receptors and targets may also be affected[18,20].
When the drug is reduced in dose or stopped, the counteractions developed in its presence persist and continue unopposed, in what might be described as hypo-serotonergic syndrome[21,22]. It is the time taken for these adaptations to resolve — which can be months or years — that may explain the long-lasting nature of withdrawal symptoms[18]. This is not determined by the time taken for the drug to leave the system, as has previously been thought. Some commentators have conceptualised the protracted antidepressant syndrome as a form of long-lasting toxicity or injury, similar to tardive dyskinesia, or to a similar protracted syndrome in benzodiazepines, sometimes referred to as benzodiazepine-induced neural dysfunction[23,24].
Reasons to stop
There are many reasons to stop antidepressants: a stressor may have resolved or the patient may have developed alternative coping skills, rendering them no longer necessary[10]. Alternatively, the medication may never have been effective (selective serotonin-reuptake inhibitors [SSRIs] have ‘numbers needed to treat’ of 7 or more), or it may have initially been effective but its effects diminished over time[25–27]. Many patients are on antidepressants for longer than guidelines recommend: NICE recommends several months of treatment for a single episode of depression, and reserves long-term treatment for severe and recurrent disorders (these recommendations have themselves been questioned)[5,28]. An estimated 30–50% of antidepressant users have no evidence-based indication to continue them[29].
Adverse (or side) effects are also important to consider, as they can impair a patient’s quality of life: 25–80% of patients experience sexual dysfunction and about 50% experience emotional numbing or blunting[30,31]. Memory and concentration issues, tiredness and weight gain are all common with antidepressants[32–35]. Observational studies have found increased rates of stroke, falls, cardiovascular disease and premature death in antidepressant users compared with non-users, albeit with debate about the degree to which causality should be attributed to antidepressants or the conditions treated[36,37]. In a survey of psychotherapy patients, patients reported a desire to live without being dependent on a medication, concerns about long-term effects on their health, a lack of efficacy, feeling better, or planning to become pregnant as their main reasons for wishing to stop medication[38].
Withdrawal effects
Withdrawal effects include a myriad of physical and mental symptoms because of the wide-ranging effects of antidepressants on various bodily systems, including the central and peripheral nervous systems, gastrointestinal system and endocrine system[22,39].
Physical and cognitive symptoms
Physical symptoms include dizziness, headache, vertigo, brain ‘zaps’, unsteadiness on one’s feet, sensory disturbances and muscle cramps[22]. Cognitive symptoms include trouble with memory and concentration, derealisation and depersonalisation[22,39]. Although akathisia has been most often associated with long-term antipsychotic use, it can also occur in antidepressant withdrawal, characterised by restlessness — sometimes prompting patients to pace — as well as internal symptoms, such as panic or terror, which patients have described as being ‘unbearable’[40,41]. All these symptoms can last weeks, months or sometimes years[6,7].
These physical symptoms can be misdiagnosed as chronic fatigue syndrome, medically unexplained symptoms, functional neurological disorder or other neurological or psychiatric conditions, because of overlap with the diagnostic criteria of these syndromes[6]. Sometimes, patients are accused of malingering or diagnosed with somatisation or delusional disorder, because clinicians are not familiar with withdrawal symptoms[6]. Antidepressant withdrawal syndrome can be distinguished from these other diagnoses because it occurs after reduction or cessation of an antidepressant, with well-characterised symptomatology, and often improves if the drug is reinstated[22,39]. Some of these misdiagnosed syndromes are diagnoses of exclusion, which is not appropriate if antidepressant withdrawal is a likely cause.
Psychological symptoms
Psychological symptoms of withdrawal include anxiety, depressed mood, panic attacks, tearfulness, crying spells, obsessive thinking and suicidality. These can all occur, even in people without underlying mental health conditions; for instance, those prescribed antidepressants for pain or the menopause. These symptoms can easily be misdiagnosed as relapse of an underlying condition or onset of a new mental health condition, a phenomenon widely reported by patients[9,42]. These symptoms can also persist for weeks, months and even years, which can be perplexing to clinicians not aware that the patient may be experiencing antidepressant withdrawal symptoms [6,7].
Although withdrawal effects usually begin days after reducing dose or stopping an antidepressant, they can be delayed. In one case–series analysis, the mean time to onset of withdrawal symptoms after stopping an SSRI was 4.5 weeks, with a standard deviation of 13 weeks, meaning some patients experienced onset of withdrawal effects a full 4 months after stopping[43]. For fluoxetine, which has an effective half-life of 1–2 weeks, this may be explained by the time required for elimination. Some have suggested that, for other SSRIs, along with serotonin and norepinephrine reuptake inhibitors (SNRIs) (with much shorter half-lives than fluoxetine), this phenomenon may be explained by a delay in washout from the central compartment for antidepressants[44]. Delayed onset withdrawal effects are commonly observed in clinical practice[43].
Feedback from patients
Tens of thousands of patients pursue help in online peer forums, dissatisfied with — or even traumatised by — the help provided by clinicians[8,9,45]. Patients report that they are taken off antidepressants too quickly by the NHS — usually over either two or four weeks — leading to withdrawal symptoms that are commonly misdiagnosed as relapse[8,9,45]. This diagnosis often leads to advice to continue antidepressants in the long term[8,9,45].
These patients request two main things from the healthcare system:
- Access to knowledgeable clinicians, who can guide them to taper in a personalised, gradual way;
- Access to formulations of medication that allow for small dose reductions, such as liquids or compounded low-dose tablets[8,9,46].
How to taper antidepressants
There has been a step-change in guidance on how to safely stop antidepressants in the past few years[47,48]. Until 2022, NICE had recommended that antidepressants should be stopped over a four-week period. This guidance had not been based on empirical studies but on expert consensus[49]. The most common approach employed by clinicians is to halve the dose of antidepressant for two weeks and then to halve it again (sometimes by advising that a tablet is taken every second day), before stopping[9]. This method is more often unsuccessful than successful. In one study, only 38% of people on antidepressants for more than one year who were motivated to stop were able to do so using this approach[50].
NICE guidelines have since been updated to reflect a new approach, known as hyperbolic tapering[5,10,22]. The relationship between the dose of an antidepressant and its effect on target receptors (e.g. inhibition of the serotonin transporter) is hyperbolic, according to the law of mass action, and evident on nuclear imaging[44,51,52]. When small amounts of drug are present, most receptors are unsaturated, and each additional milligram of drug has a large additive effect. When larger amounts are present (e.g. at clinically-employed doses), most receptors are saturated, and each additional milligram has incrementally less effect. For example, 2mg of citalopram has half the effect on serotonin transporter inhibition as 40mg[22].
As a consequence of this hyperbolic relationship, linear reductions in dose (e.g. 20mg, 15mg, 10mg, 5mg, 0mg) will cause increasingly large reductions in the effect on target receptors, which corresponds to increasingly severe withdrawal effects (see Figure 1a)[22,53]. Instead, reducing dose in a manner that causes an ‘even’ reduction in effect on target receptors requires hyperbolic tapering, where the reductions are made by smaller and smaller amounts (Figure 1b)[22].
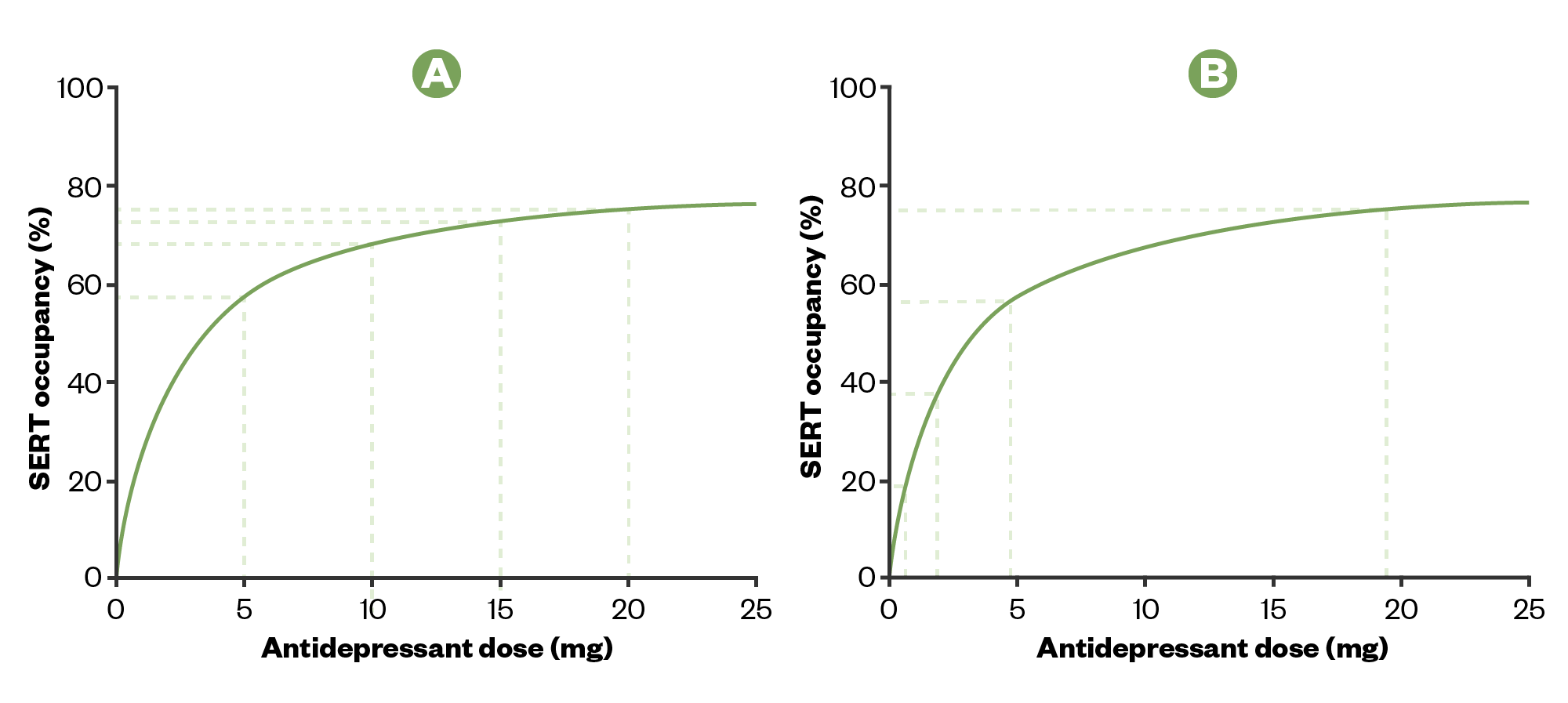
The relationship between the dose of an antidepressant and its effect on target receptors is hyperbolic.
A) Linear reductions of dose cause increasingly large reductions in effect on receptor targets, associated with more withdrawal effects.
B) ‘Even’ reductions of effect at target receptors require hyperbolic dose reductions. The final dose before stopping will need to be very small so that this step down is not larger (in terms of effect on receptor occupancy) than previous reductions.
SERT: serotonin transporter
The Pharmaceutical Journal
NICE guidelines now recommend dose reductions be made in a size that is “in proportion” to the most recent dose, so that the size of the reductions gets smaller and smaller as the dose gets lower — a simple approximation of hyperbolic reductions[5,10]. The guideline specifies that tapering antidepressants may take months, and that the rate of tapering should be adjusted so that withdrawal effects are tolerable for a particular patient[5].
Importantly, the guideline signals a change in policy with respect to liquid versions of medication, which in the past may have been discouraged for cost reasons. It advises that “if, once very small doses have been reached, slow tapering cannot be achieved using tablets or capsules, consider using liquid preparations if available”[5]. Although the guidance provided is brief, NICE also links to Royal College of Psychiatrists guidance on stopping antidepressants, which provides more detailed information[48].
NICE does not recommend switching other antidepressants to fluoxetine before tapering[5]. This approach was first suggested by the manufacturer of fluoxetine when a trial demonstrated less severe withdrawal effects for fluoxetine than other antidepressants during a one-week washout period[54]. However, this switching approach has not been demonstrated empirically to be effective. Fluoxetine’s long half-life means that a week is not a sufficient period to observe withdrawal effects that are often delayed in onset and more frequent than generally supposed[18,55]. The approach is derived from that of benzodiazepines, where a switch to diazepam is often recommended; however, the pharmacodynamic targets of benzodiazepines are more similar as a group than those of SSRIs and SNRIs. A switch to fluoxetine may still be considered as an approach to tapering antidepressants if no other options seem viable; although, in clinical practice, it has mixed results.
Tapering in practice
Community and primary care pharmacists can advise and support patients in the process of tapering. Many clinicians attempt to reduce doses by extending the dosing interval (e.g. ‘every-other-day’ dosing). Most antidepressants have half-lives of 24 hours or less; every-other-day dosing will lead to trough levels that are a quarter of peak levels, which can trigger withdrawal symptoms in susceptible patients[56]. It is generally better to provide a smaller dose each day than extend the dosing interval.
Patients should be made aware by their prescriber or a pharmacist that several options are available to make up smaller doses. Many antidepressants come as liquid formulations in the UK. Some liquid versions come with a dropper mechanism that delivers, for example, 2mg per drop. To make up small doses, the dropper mechanism can be removed and a small syringe (e.g. measuring 1mL) used instead. Highly concentrated solutions will require dilution in water to measure small doses. Compounded medication can be an option[57].
Many tablets can be divided into halves (or quarters if they are round) using cheap tablet cutters[58]. Using tablet cutters is more accurate for dividing tablets than either splitting by hand (for scored tablets), cutting with scissors (for unscored tablets) or using a kitchen knife[59]. This technique may be helpful for the first few steps of a reduction regimen but may not be suitable after this stage as the doses required will be smaller than one quarter of the smallest available tablet for some medications. If tablets crumble then this approach will not be suitable. Sustained- and extended-release tablets can be split with the understanding that splitting will compromise their slow-release properties; therefore they should be treated as immediate-release formulations.
Well-known off-label options are also available, supported by the General Medical Council and the Royal Pharmaceutical Society, when no licensed alternatives are available[58,60]. Tablets can be crushed to make up suspensions, as advised by the NHS Specialist Pharmacy Service and the NEWT guidelines for people who cannot swallow tablets[61,62]. For example, a patient can be shown how a 50mg sertraline tablet can be crushed with a spoon and made up to 50mL with water to make a 1mg/mL suspension. This should be shaken vigorously before use so that the drug is adequately mixed throughout and allows small doses to be easily measured with a syringe. As its stability cannot be assured, the suspension should be discarded after use and made up freshly each day.
Medications such as venlafaxine and duloxetine, which come as capsules containing slow-release beads, can be opened and the beads counted or weighed. The manufacturer of duloxetine has demonstrated that these beads are stable in air, although it is an off-label use. This approach is commonly taken by patients in peer-led tapering communities[56,63]. Patients can be shown how to carefully open a capsule onto a piece of soft material so that beads do not bounce. Beads can be counted in groups of five to make up the amount required before placing them back into the gelatine capsule for consumption (beads can irritate the throat if swallowed without a capsule). Various guides for counting and weighing are available online, including directions for the use of a jeweller’s scale[64].
Case examples
The following cases demonstrate common scenarios that a pharmacist may encounter in primary or secondary care, in which recognition of withdrawal effects and advice about safe tapering would be useful. The importance of access to formulations of medication that allow easy manipulation of dose is highlighted throughout.
Case 1
A GP asks you as the primary care pharmacist to counsel a man aged 59 years who has used citalopram for 20 years at a dose of 20mg and has asked for advice on how to stop his medication. His motivation for doing so is he feels “foggy” and tired during the day, and suspects that the medication is the cause. He has tried to stop four times previously and had not had trouble getting down to a dose of 5mg, but on stopping each time from this dose, he experienced panic attacks, trouble sleeping and felt suicidal. A previous GP told him that this experience indicated that he must need the medication to stay well but he is unsure as he does not recall ever having these symptoms before starting the drug. He started on the medication because he was depressed after being made redundant from his job. You advise him that he is in a high-risk category for withdrawal effects and will need to taper in a gradual, hyperbolic manner (you show him Figure 1)[18]. This may take several months or more than a year, but is likely to avoid prompting the severe symptoms he has experienced in the past.
You give him the guide in Table 1 and arrange to check in on him each month[65]. You show him how to make halves and quarters of his round tablets with a tablet cutter. His GP prescribes a liquid version of citalopram to use for doses less than 5mg. As the dropper mechanism delivers 2mg doses per drop, you show the patient how to remove the dropper mechanism and use a small syringe instead. Citalopram drops are very concentrated (40mg/mL) and so a dilution is required to make up smaller doses. The manufacturer advises that the drops can be mixed with water or juice[66]. A 10-fold dilution can be made using 0.5mL of the original solution mixed with 4.5mL of water to make up a 4mg/mL solution. These solutions should be shaken vigorously before use and, as their stability cannot be assured, made up freshly each day. An alternative to make up smaller doses is to put 1 drop in some water and drink a portion. For example, 1mg can be measured by putting 1 drop (2mg) in 20mL of water, mixing well and drinking 10mL of the solution.
Citalopram solution contains 25% more bio-available citalopram than the solid because the liquid contains citalopram hydrochloride, rather than the hydrobromide salt in the tablet. This means that 8mg of citalopram solution is equivalent to 10mg in tablet form. The regimen provided can be slowed down if the patient finds withdrawal symptoms unpleasant by adding in further intermediate steps[65].
Case 2
A woman aged 32 years, who has used sertraline 100mg for 10 years, comes into the community pharmacy in distress. She says that she is having trouble sleeping, has developed obsessive thinking about odd and distressing topics, and feels very anxious. She stopped her sertraline 100mg about 2 months ago by halving the dose for 2 weeks and then taking 50mg every second day for another 2 weeks, before completely stopping. She wanted to stop the medication because she is planning a pregnancy later in the year. On further questioning, it is revealed she has also felt dizzy, been unsteady on her feet and has headaches. All these symptoms came on 4 weeks after stopping her medication and have gotten worse. She was put on the medication during a tumultuous period in her early 20s, and says it was helpful initially, but the effects wore off after a few months.
She says that the obsessive thinking, dizziness and unsteadiness on her feet are all new to her. You advise her that the co-occurrence of physical symptoms with psychological symptoms, and the distinction from her previous psychological symptoms, means the most likely diagnosis is a withdrawal syndrome. You explain that sometimes these symptoms can begin weeks after stopping.
You advise her to return to 50mg of sertraline and within 2 weeks she feels better, although it takes her another 6 weeks to feel back to baseline. She asks you if it is possible to safely stop this medication. You explain to her that gradual, hyperbolic tapering can make withdrawal symptoms more tolerable.
Based on her risk factors for withdrawal and her desire to stop this medication to become pregnant, you start her on a ‘faster’ regimen (see Table 2)[18,65]. Unfortunately, there are no liquid versions of sertraline currently available, so you explain how to make a safe suspension by crushing a tablet and mixing it with water. If she experiences unpleasant withdrawal symptoms, this rate of reduction can be slowed by inserting intermediate steps[65].
Case 3
A 25-year-old man asks for your advice in a community pharmacy about how to safely stop venlafaxine 75mg, which he has been on for 3 years. He was originally put on this medication after his mother passed away. He notices when he misses a dose that he gets brain ‘zaps’ and a headache. When he forgot to take the medication on holiday once he was forced to return home after 4 days because he was so unwell — he felt nauseous, unsteady on his feet, and like the world around him was not real (he describes it as “dream-like”). He does not want to be on a medication that does this to him — he says that he is happy to take as long as is required to get off the medication and has read that this is a particularly difficult drug to stop. He had asked his GP for a liquid version, but was told it was too expensive to prescribe. He wonders if there is any other way to stop venlafaxine.
You explain to him that it would be worth approaching his GP again, perhaps showing the NICE or Royal College of Psychiatrists guidelines that recommend a liquid version of the drug, or approaching another GP. If he is unable to obtain this formulation, then it is possible to open up the capsule and to count (or weigh) beads. As a starting point, he could remove a third of the beads. Based on the degree of withdrawal symptoms, this rate could be adjusted so that the process is tolerable. It is not unusual for it to take more than a year to safely stop venlafaxine after long-term use. The 75mg capsules contain about 200 beads and he reduces down to 133 beads as a first reduction, with mild and tolerable symptoms. He continues reducing his dose following the regimen in Table 3[65].
Conclusion
The healthcare system has been slow to recognise the sometimes severe and long-lasting symptoms that withdrawing from antidepressants can cause patients. These symptoms have often been misinterpreted as a relapse of a patient’s underlying condition. As a result, increasing numbers of patients have abandoned mainstream healthcare services to receive guidance and support from social media sites. These patients have a clear request for clinicians to taper medications more gradually and carefully, especially at lower doses, to avoid severe and long-lasting withdrawal effects. Newly updated NICE and Royal College of Psychiatrists guidelines recommend hyperbolic/proportional tapering (which benefits both patients and the healthcare system), conducted gradually and titrated to the individual, consistent with these requests from patients; however, implementation has been variable. One major barrier to widespread use of more careful hyperbolic tapering is the complexities of using formulations other than tablets. Pharmacists have an opportunity to play a central role in providing advice to GPs and patients about best practice in these areas, to help optimise patient care by reducing inappropriate long-term prescribing and avoiding unnecessary harm caused by potentially harmful methods of discontinuation.
- 1Prescribed medicines review: report. Public Health England. 2020.https://www.gov.uk/government/publications/prescribed-medicines-review-report (accessed Sep 2023).
- 2Position statement on antidepressants and depression. Royal College of Psychiatrists. 2019.https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps04_19—antidepressants-and-depression.pdf?sfvrsn=ddea9473_5 (accessed Sep 2023).
- 3Iacobucci G. NICE updates antidepressant guidelines to reflect severity and length of withdrawal symptoms. BMJ. 2019;:l6103. doi:10.1136/bmj.l6103
- 4Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addictive Behaviors. 2019;97:111–21. doi:10.1016/j.addbeh.2018.08.027
- 5Depression in Adults: Treatment and Management. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng222 (accessed Sep 2023).
- 6Guy A, Brown M, Lewis S, et al. The ‘patient voice’: patients who experience antidepressant withdrawal symptoms are often dismissed, or misdiagnosed with relapse, or a new medical condition. Therapeutic Advances in Psychopharmacology. 2020;10:204512532096718. doi:10.1177/2045125320967183
- 7Hengartner MP, Schulthess L, Sorensen A, et al. Protracted withdrawal syndrome after stopping antidepressants: a descriptive quantitative analysis of consumer narratives from a large internet forum. Therapeutic Advances in Psychopharmacology. 2020;10:204512532098057. doi:10.1177/2045125320980573
- 8Read J, Lewis S, Horowitz M, et al. The need for antidepressant withdrawal support services: Recommendations from 708 patients. Psychiatry Research. 2023;326:115303. doi:10.1016/j.psychres.2023.115303
- 9Read J, Moncrieff J, Horowitz MA. Designing withdrawal support services for antidepressant users: Patients’ views on existing services and what they really need. Journal of Psychiatric Research. 2023;161:298–306. doi:10.1016/j.jpsychires.2023.03.013
- 10Medicines associated with dependence or withdrawal symptoms: safe prescribing and withdrawal management for adults. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ng215/chapter/Recommendations (accessed Sep 2023).
- 11Horowitz MA, Taylor D. Addiction and physical dependence are not the same thing. The Lancet Psychiatry. 2023;10:e23. doi:10.1016/s2215-0366(23)00230-4
- 12O’Brien C. Addiction and dependence in DSM-V. Addiction. 2010;106:866–7. doi:10.1111/j.1360-0443.2010.03144.x
- 13Brunton L, Chabner B, Knollmann B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill Education 2011.
- 14Massabki I, Abi-Jaoude E. Selective serotonin reuptake inhibitor ‘discontinuation syndrome’ or withdrawal. Br J Psychiatry. 2020;218:168–71. doi:10.1192/bjp.2019.269
- 15Optimising personalised care for adults prescribed medicines associated with dependence or withdrawal symptoms: Framework for action for integrated care boards (ICBs) and primary care. NHS England. 2023.https://www.england.nhs.uk/long-read/optimising-personalised-care-for-adults-prescribed-medicines-associated-with-dependence-or-withdrawal-symptoms/ (accessed Sep 2023).
- 16National Medicines Optimisation Opportunities 2023/2024. NHS England. 2023.https://www.england.nhs.uk/long-read/national-medicines-optimisation-opportunities-2023-24/#7-addressing-inappropriate-antidepressant-prescribing (accessed Sep 2023).
- 17Pharmacist prescribing pilots to test new services for depression and minor illnesses. Pharmaceutical Journal. 2023. doi:10.1211/pj.2023.1.184635
- 18Horowitz MA, Framer A, Hengartner MP, et al. Estimating Risk of Antidepressant Withdrawal from a Review of Published Data. CNS Drugs. 2022;37:143–57. doi:10.1007/s40263-022-00960-y
- 19Bhagwagar Z, Rabiner EA, Sargent PA, et al. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–92. doi:10.1038/sj.mp.4001401
- 20Renoir T. Selective Serotonin Reuptake Inhibitor Antidepressant Treatment Discontinuation Syndrome: A Review of the Clinical Evidence and the Possible Mechanisms Involved. Front. Pharmacol. 2013;4. doi:10.3389/fphar.2013.00045
- 21Reidenberg MM. Drug Discontinuation Effects Are Part of the Pharmacology of a Drug. J Pharmacol Exp Ther. 2011;339:324–8. doi:10.1124/jpet.111.183285
- 22Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. The Lancet Psychiatry. 2019;6:538–46. doi:10.1016/s2215-0366(19)30032-x
- 23Fava GA, Cosci F. Understanding and Managing Withdrawal Syndromes After Discontinuation of Antidepressant Drugs. J. Clin. Psychiatry. 2019;80. doi:10.4088/jcp.19com12794
- 24Ritvo AD, Foster DE, Huff C, et al. Long-term consequences of benzodiazepine-induced neurological dysfunction: A survey. PLoS ONE. 2023;18:e0285584. doi:10.1371/journal.pone.0285584
- 25Arroll B, Elley CR, Fishman T, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Systematic Reviews. 2009. doi:10.1002/14651858.cd007954
- 26Munkholm K, Paludan-Müller AS, Boesen K. Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta-analysis. BMJ Open. 2019;9:e024886. doi:10.1136/bmjopen-2018-024886
- 27Kinrys G, Gold AK, Pisano VD, et al. Tachyphylaxis in major depressive disorder: A review of the current state of research. Journal of Affective Disorders. 2019;245:488–97. doi:10.1016/j.jad.2018.10.357
- 28Hengartner MP. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Therapeutic Advances in Psychopharmacology. 2020;10:204512532092169. doi:10.1177/2045125320921694
- 29Cruickshank G, Macgillivray S, Bruce D, et al. Cross-sectional survey of patients in receipt of long-term repeat prescriptions for antidepressant drugs in primary care. Ment Health Fam Med 2008;5:105–9.https://www.ncbi.nlm.nih.gov/pubmed/22477855
- 30Serretti A, Chiesa A. Treatment-Emergent Sexual Dysfunction Related to Antidepressants. Journal of Clinical Psychopharmacology. 2009;29:259–66. doi:10.1097/jcp.0b013e3181a5233f
- 31Ma H, Cai M, Wang H. Emotional Blunting in Patients With Major Depressive Disorder: A Brief Non-systematic Review of Current Research. Front. Psychiatry. 2021;12. doi:10.3389/fpsyt.2021.792960
- 32Hindmarch I. Cognitive toxicity of pharmacotherapeutic agents used in social anxiety disorder. International Journal of Clinical Practice. 2009;63:1085–94. doi:10.1111/j.1742-1241.2009.02085.x
- 33Read J, Williams J. Adverse Effects of Antidepressants Reported by a Large International Cohort: Emotional Blunting, Suicidality, and Withdrawal Effects. CDS. 2018;13:176–86. doi:10.2174/1574886313666180605095130
- 34Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;:k1951. doi:10.1136/bmj.k1951
- 35Horowitz M, Wilcock M. Newer generation antidepressants and withdrawal effects: reconsidering the role of antidepressants and helping patients to stop. DTB. 2021;60:7–12. doi:10.1136/dtb.2020.000080
- 36Bansal N, Hudda M, Payne RA, et al. Antidepressant use and risk of adverse outcomes: population-based cohort study. BJPsych open. 2022;8. doi:10.1192/bjo.2022.563
- 37Kendrick T. Strategies to reduce use of antidepressants. Brit J Clinical Pharma. 2020;87:23–33. doi:10.1111/bcp.14475
- 38Horowitz M, Moncrieff J. Antidepressant withdrawal symptoms reported by patients enrolled in a public psychotherapy service. Unpublished. 2023._ (accessed Sep 2023).
- 39Fava GA, Gatti A, Belaise C, et al. Withdrawal Symptoms after Selective Serotonin Reuptake Inhibitor Discontinuation: A Systematic Review. Psychother Psychosom. 2015;84:72–81. doi:10.1159/000370338
- 40Akathisia: General Information. Akathisia Alliance. 2023.https://akathisiaalliance.org/about-akathisia/ (accessed Sep 2023).
- 41Wolfe R. Antidepressant withdrawal reactions. Am Fam Physician 1997;56:455–62.https://www.ncbi.nlm.nih.gov/pubmed/9262526
- 42Horowitz MA, Taylor D. Distinguishing relapse from antidepressant withdrawal: clinical practice and antidepressant discontinuation studies. BJPsych advances. 2022;28:297–311. doi:10.1192/bja.2021.62
- 43Stockmann T, Odegbaro D, Timimi S, et al. SSRI and SNRI withdrawal symptoms reported on an internet forum. JRS. 2018;29:175–80. doi:10.3233/jrs-180018
- 44Sørensen A, Ruhé HG, Munkholm K. The relationship between dose and serotonin transporter occupancy of antidepressants—a systematic review. Mol Psychiatry. 2021;27:192–201. doi:10.1038/s41380-021-01285-w
- 45White E, Read J, Julo S. The role of Facebook groups in the management and raising of awareness of antidepressant withdrawal: is social media filling the void left by health services? Therapeutic Advances in Psychopharmacology. 2021;11:204512532098117. doi:10.1177/2045125320981174
- 46Cooper RE, Ashman M, Lomani J, et al. “Stabilise-reduce, stabilise-reduce”: A survey of the common practices of deprescribing services and recommendations for future services. PLoS ONE. 2023;18:e0282988. doi:10.1371/journal.pone.0282988
- 47Palmer EG, Sornalingam S, Page L, et al. Withdrawing from SSRI antidepressants: advice for primary care. Br J Gen Pract. 2023;73:138–40. doi:10.3399/bjgp23x732273
- 48Stopping antidepressants. Royal College of Psychiatrists. 2020.https://www.rcpsych.ac.uk/mental-health/treatments-and-wellbeing/stopping-antidepressants (accessed Sep 2023).
- 49National Collaborating Centre for Mental Health (UK). nicecg90. Published Online First: 1 January 2010.http://www.ncbi.nlm.nih.gov/books/NBK63748/
- 50Hajisadeghi M. REDUCE study results, Allam Lecture. Twitter. 2023.https://twitter.com/Mahe_Sadeghi/status/1663937827984269313 (accessed Sep 2023).
- 51Holford N. Pharmacodynamic principles and the time course of delayed and cumulative drug effects. Transl Clin Pharmacol. 2018;26:56. doi:10.12793/tcp.2018.26.2.56
- 52Meyer JH, Wilson AA, Sagrati S, et al. Serotonin Transporter Occupancy of Five Selective Serotonin Reuptake Inhibitors at Different Doses: An [11C]DASB Positron Emission Tomography Study. AJP. 2004;161:826–35. doi:10.1176/appi.ajp.161.5.826
- 53van Os J, Groot PC. Outcomes of hyperbolic tapering of antidepressants. Therapeutic Advances in Psychopharmacology. 2023;13:204512532311715. doi:10.1177/20451253231171518
- 54Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biological Psychiatry. 1998;44:77–87. doi:10.1016/s0006-3223(98)00126-7
- 55Gastaldon C, Schoretsanitis G, Arzenton E, et al. Withdrawal Syndrome Following Discontinuation of 28 Antidepressants: Pharmacovigilance Analysis of 31,688 Reports from the WHO Spontaneous Reporting Database. Drug Saf. 2022;45:1539–49. doi:10.1007/s40264-022-01246-4
- 56Framer A. What I have learnt from helping thousands of people taper off antidepressants and other psychotropic medications. Therapeutic Advances in Psychopharmacology. 2021;11:204512532199127. doi:10.1177/2045125321991274
- 57Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Therapeutic Advances in Psychopharmacology. 2020;10:204512532093245. doi:10.1177/2045125320932452
- 58Pharmaceutical Issues when Crushing, Opening or Splitting Oral Dosage Forms. Royal Pharmaceutical Society. 2011.https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support/toolkit/pharmaceuticalissuesdosageforms-%282%29.pdf (accessed Sep 2023).
- 59Verrue C, Mehuys E, Boussery K, et al. Tablet-splitting: a common yet not so innocent practice. Journal of Advanced Nursing. 2010;67:26–32. doi:10.1111/j.1365-2648.2010.05477.x
- 60Prescribing unlicensed medicines. General Medical Council. 2021.https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/good-practice-in-prescribing-and-managing-medicines-and-devices/prescribing-unlicensed-medicines (accessed Sep 2023).
- 61The NEWT Guidelines for Administration of Medication to Patients with Enteral Feeding Tubes or Swallowing Difficulties. Wrexham Maelor Hospital. 2011.https://www.newtguidelines.com/index.html (accessed Sep 2023).
- 62SSRI suggestions for adults with swallowing difficulties. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/selective-serotonin-reuptake-inhibitor-ssri-formulations-suggested-for-adults-with-swallowing-difficulties/ (accessed Sep 2023).
- 63Wells KA, Losin WG. In vitro stability, potency, and dissolution of duloxetine enteric-coated pellets after exposure to applesauce, apple juice, and chocolate pudding. Clinical Therapeutics. 2008;30:1300–8. doi:10.1016/s0149-2918(08)80054-9
- 64Tapering Slowly, Steadily and Consistently off Cymbalta/Duloxetine. Cymbalta Hurts Worse. 2019.http://www.healingamericanow.com/chw-tapering-guide/ (accessed Sep 2023).
- 65Horowitz M, Taylor D. The Maudsley Deprescribing Guidelines in Psychiatry: Antidepressants, Benzodiazepines, Gabapentinoids and Z-drugs. Unpublished. 2023._ (accessed Sep 2023).
- 66Citalopram 40mg/ml Oral Drops, solution. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/emc/product/3349/smpc (accessed Sep 2023).
2 comments
You must be logged in to post a comment.
You might also be interested in…
GPhC writes to pharmacy teams after methotrexate dispensed with instruction to take once daily

Medicines commission calls for greater clarity on risk of suicidal behaviour from antidepressants

I have been on Effexor for 30 years and am hoping to get off it in one or two years by taking the drug in compounded form with tiny dosage decreases that occur every few days or so. Such an approach makes perfect psychological sense to me but I don't know anyone who's doing it. I think it's absurd that users trying to withdraw should have to be counting out pill pellets. That's what pharmacist are paid to do, and with the appropriate equipment. Also, regarding why people might want to stop using such drugs, here's one that no one ever mentions: the fact that it is humiliating to become an eternal patient and have to visit a "behavioral health clinic" every three months to have your refill approved by someone who's half or even one-third your own age. Such drugs turn users into eternal patient. That's the main reason I want to quit them in my retirement years.
Unfortunately, only compounding pharmacies can perform dose manipulation (in reality). Wish extemporaneous preparation was still a thing in community.