Sovereign, ISM / Science Photo Library
Chronic oedema is increasingly recognised as a common condition and can be defined as soft tissue swelling that has been present for three months or more[1]
. Chronic oedema is more frequently seen in those aged over 65 years, in women and in obese patients, with the number affected likely to increase along with the increasing levels of obesity[2]
. Swelling, usually of the legs, leads to distortion of the shape of the limb, skin changes, fluid leakage and longer term complications, such as cellulitis and ulceration.
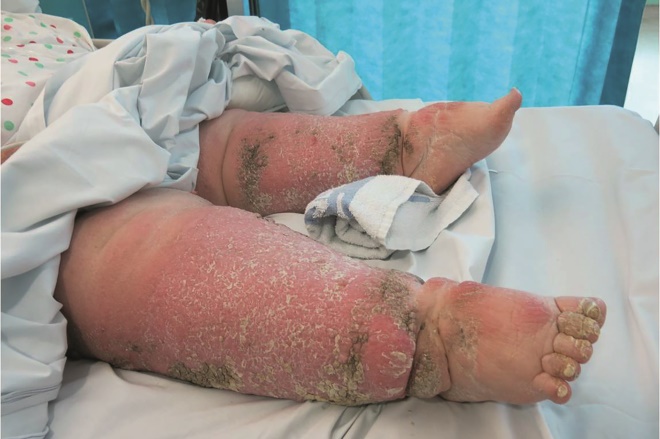
Figure 1: Oedematous legs
Source: Courtesy of Madeleine Flanagan
Chronic oedema with associated skin changes; skin folds, fibrosclerotic tissue and chronic inflammation are clearly visible
In 2000, a study of healthcare professionals from dedicated lymphoedema services, specific outpatient clinics, hospital wards and community services in south-west London, identified 823 patients with chronic oedema; 83% were female with a prevalence that rose from 1.3 in 1,000 patients across all age groups, to around 1 in 200 in those aged over 65 years and 1 in 100 in those aged over 85 years[3]
. Other study data are scarce, but results from a Derby Hospital survey study from 2012 showed that around 25% of all inpatient respondents had some degree of chronic oedema[4]
.
Unfortunately, the presence of chronic oedema is not always taken into consideration when prescribing medicines in patients with multiple morbidities. This can lead to patients with chronic oedema inadvertently being prescribed a medicine that worsens it; for example, those with diabetes being prescribed a thiazolidinedione or dipeptidylpeptidase-4 inhibitor.
This article aims to highlight medicines that can cause or worsen chronic oedema, to describe treatment options and consider how pharmacists and healthcare professionals can contribute to the care of patients with lower-leg oedema. It is therefore important to understand the process of oedema formation and the mechanisms by which medicines can influence this.
Oedema formation
Oedema is the term used to describe the accumulation of fluid in the interstitial space. The human body is around 60% water by weight[5]
and this can be found either within the cells (intracellular) or between the cells (extracellular)[6]
.
The transcapillary movement of fluid between plasma and the interstitial space, controlled by both hydrostatic and oncotic pressure, was described by Starling in 1896[7]
. Hydrostatic pressure is produced by the fluid pressure of the blood volume entering the capillaries, and oncotic pressure is generated by proteins within the plasma[7]
. In a healthy individual, hydrostatic pressure is greater than oncotic pressure at the arterial or pre-capillary end, thus fluid moves into the interstitial space. At the venous or post-capillary end, where oncotic pressure is greater than hydrostatic pressure, the opposite was thought to be true and fluid was believed to move back into the capillaries. Excess interstitial fluid is then drained away via the lymphatic system[8]
.
However, it has now been established that there is no sustained venous reabsorption of interstitial fluid. Thus, the fluid filtered from blood vessels is reabsorbed by lymphatic vessels (except for minor transient periods of reabsorption)[9]
. Therefore, all chronic oedema represents lymph drainage failure, either because the lymphatic system cannot cope owing to high lymph load (increased fluid entry), or a fault in the lymphatic system (reduced outflow), or both.
Increased fluid entry into the interstitial space
This may be a result of an increase in hydrostatic pressure, or an increase in capillary permeability. Conditions such as heart failure can lead to fluid overload and an increase in hydrostatic pressure, while allergic reactions and local infection can increase capillary permeability[6]
.
Reduced outflow of fluid from the interstitial space
As it has now been demonstrated, tissue fluid balance depends upon lymphatic function in most tissues[10]
. A decrease in lymphatic drainage resulting in oedema may be primary (genetic), or secondary to damage to the lymphatic system. Decreases are most commonly caused by tumour, surgery, radiation therapy and infection[11]
.
A vicious cycle
The unfortunate result of fluid accumulating in the interstitial space in the form of oedema is a reduction in circulatory volume, which activates the renin-angiotensin-aldosterone system, promoting sodium and water retention and further increasing the plasma volume[6]
(see Figure 2).
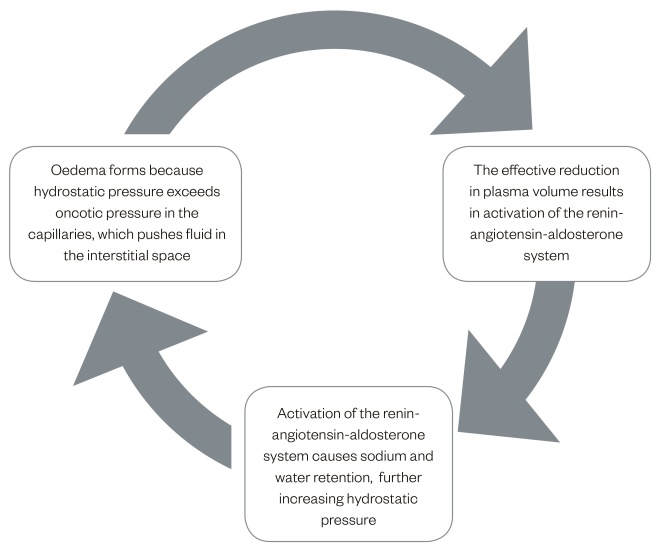
Figure 2: Cycle of oedema formation
The formation of oedema begins when hydrostatic pressure exceeds oncotic pressure. The resultant stages are described above.
How medicines cause oedema
Increased hydrostatic pressure
Drugs that cause vasodilation or fluid retention can increase hydrostatic pressure, causing an increase in movement of fluid into the interstitial space. This results in oedema if the capacity of the lymphatic system to drain away the fluid is overwhelmed. Calcium channel blockers (CCBs) act to increase blood flow into the capillaries as a result of dilation of the small arteries that carry blood to the capillaries[12]
. CCBs have also been implicated in reducing lymph drainage (see ‘Decrease in lymphatic drainage’). Corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), sex hormones and related compounds (e.g. raloxifene and megestrol) result in fluid retention[12]
.
Increased capillary permeability
Capillary leak syndrome (CLS) has been reported with filgrastim and pegfilgrastim[13]
. Symptoms of CLS include hypotension, oedema, hypoalbuminaemia and haemoconcentration. CLS may be fatal unless promptly diagnosed and managed[13]
.
Decrease in lymphatic drainage
It has been suggested that CCBs reduce lymph drainage by decreasing the spontaneous contraction of smooth muscle within the lymph vessels[4]
, but a more recent study with nifedipine suggests that while this may occur in vitro, it does not occur in vivo
[14]
.
Medicines that cause oedema
Many medicines can cause or worsen oedema; CCBs and NSAIDs are among the most commonly implicated[6]
. Table 1 lists some of the medicines most frequently reported and the method by which they cause oedema; these are discussed further below.
| Drug class | Mechanism |
|---|---|
| Calcium channel blockers | Increased hydrostatic pressure resulting from an increase in capillary blood flow as a result of dilation of the small arteries |
| Corticosteroids | Increased hydrostatic pressure resulting from fluid retention |
| Non-steroidal anti-inflammatories | |
| Sex hormones and related compounds |
Calcium channel blockers
Peripheral oedema is a recognised adverse effect of CCBs[15]
with up to 50% of patients on CCBs developing oedema[16]
. Female gender, increasing age, heart failure, upright postures and warm environment appear to increase the risk of developing ankle oedema while using CCBs and the frequency with which peripheral oedema occurs is both compound-specific and dose-dependent[17]
. Table 2 indicates the frequency with which peripheral oedema occurs with some of the CCBs. The UK Medicines Information Service provides more detailed information[18]
. Table 3, which gives the frequency of oedema seen with different doses of the CCBs amlodipine and felodipine, illustrates how oedema is dose-dependent.
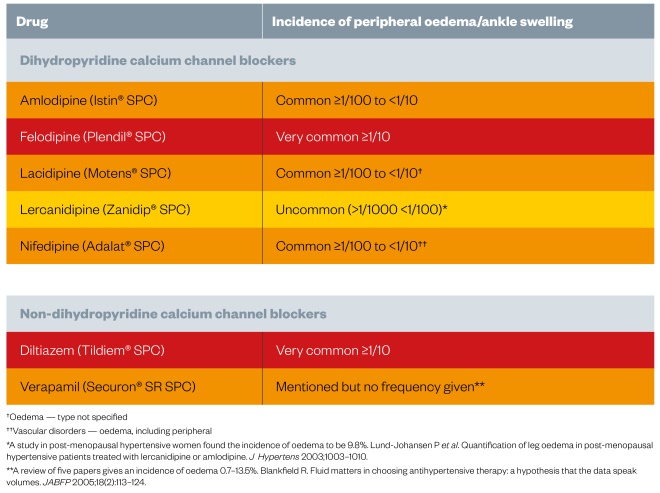
Table 2: Incidence of peripheral oedema with calcium channel blockers[19]
,[20]
,[21]
,[22]
,[23]
,[24]
,[25]
| Drug | Daily dose | Frequency of oedema (%) |
|---|---|---|
| Source: Adapted from Keeley[4] | ||
| Amlodipine | 2.5mg 5mg 10mg | 1.8 3.0 10.8 |
| Felodipine | 2.5mg 5mg 10mg | 2.0 8.8 17.4 |
Corticosteroids
Sodium and water retention[15]
caused by corticosteroids is a recognised cause of peripheral oedema[6]
. This effect is dependent upon both the dose and the duration of treatment. Patients receiving a short-term course (one to two weeks) for asthma tend not to develop much oedema, while patients on long-term treatment may experience significant swelling[4]
.
NSAIDs
NSAIDs commonly cause fluid retention[26]
, although there are differences in the frequency with which this occurs between different drugs[12]
. There are also concerns regarding the risk of necrotising fasciitis and dermal infection with the use of NSAIDs[27]
,[28]
, which is relevant to this group of patients (see ‘Analgesia’).
Sex hormones and related compounds
Anti-oestrogens and aromatase inhibitors, common sex hormones and related compounds may cause oedema because of fluid retention[12]
. One difficulty is that several of these medicines are used in the management of patients with breast cancer, who may have had a partial or radical mastectomy with or without removal of lymph nodes and who are at high risk of developing lymphoedema. It may be necessary to weigh up the risk/benefit ratio in these patients, or to consider an alternative.
Other drugs that can cause oedema
Box 1 lists some other frequently prescribed medicines that are known to cause oedema, but this list is not exhaustive. If pharmacists or healthcare professionals suspect that a medicine may be causing oedema, it would be advisable to check the summary of product characteristics (SPC) and highlight any concerns to the clinician managing the patient.
Although peripheral oedema is an uncommon side effect of proton pump inhibitors, they have been included because of the high frequency with which they are prescribed. For many of these drugs the exact mechanism by which they cause oedema is unknown.
Box 1: Other frequently prescribed drugs known to cause peripheral oedema/oedema*
Alpha blockers
- Doxazosin (common)[29]
Anticholinergics
- Tolterodine (common)[30]
Anticonvulsants
Antidepressants
Monoamine oxidase inhibitors (MAOIs)
Other
Antidiabetics
- Insulin (unspecified)[36]
- Pioglitazone (commonb)[37]
- Saxagliptin (common)[38]
- Sitagliptin (common)[39]
- Vildagliptin (common)[40]
Antiepileptics
Antifungals
- Voriconazole (very common)[43]
Antihypertensives
- Beta blockers (unspecified)[11]
- Clonidine (unspecified)[11]
- Hydralazine (‘oedema’* uncommon)[44]
- Methyldopa (unknown)[11]
- Minoxidil (unknown, ‘oedema’ common)[45]
Antipsychotics
- Lithium (unknown)[46]
- Olanzapine (‘oedema’* common)[47]
- Quetiapine (common)[48]
- Risperidone (‘oedema’c common)[49]
Bisphosphonates
Chemotherapeutic drugs
Granulocyte-colony stimulating factors
Immunosuppressants
Parkinson’s treatments
- Amantadine (very common)[59]
- Cabergoline (very common)[60]
- Pramipexole (common)[61]
- Ropinirole (common)[62]
- Rotigotine (common)[63]
Proton pump inhibitors
- Esomeprazole (uncommon)[64]
- Omeprazole (uncommon)[65]
- Pantoprazole (rare)[66]
- Rabeprazole (not known)[67]
* Oedema — unspecified
a 10% of patients (very common = ≥1/10)
b 6–9% of patients over 1 year in clinical trials (common = ≥1/100 to <1/10)
c Oedema includes: generalised oedema, oedema peripheral, pitting oedema
d 1.6% of patients in one clinical trial (common = ≥1/100 to <1/10)
e Erythematous oedema mainly of the lower limbs has been reported with an unknown frequency
Information on adverse effects is also available from the Medicines and Healthcare products Regulatory Agency (MHRA). Table 5 lists the drugs most frequently reported as suspected to have caused peripheral oedema over the five year period from December 2011 to December 2016. However, it is important to be aware that these data cannot be used to identify the drugs that most commonly cause peripheral oedema, because many factors can influence the level of reporting and reports are submitted on suspicion of an association[68]
.
| Drug substance | Number of reports |
|---|---|
| Source: MHRA | |
| Amlodipine | 90 |
| Clozapine | 33 |
| Rivaroxaban | 26 |
| Pregabalin | 21 |
| Mirtazapine | 16 |
| Adalimumab | 13 |
| Felodipine | 12 |
| Olanzapine | 12 |
| Doxazosin | 10 |
| Gabapentin | 10 |
| Prednisolone | 10 |
| Telaprevir | 10 |
It should be noted that there are several difficulties in identifying medicines that cause peripheral oedema. Part of the problem is the way in which information related to medicine side effects is recorded and interpreted. Within an SPC, the manufacturer often lists side effects that occurred at an incidence greater than is seen with placebo and in more than 1% of patients; however, the difference in incidence may be small. For example, the SPC for alendronate lists peripheral oedema in the tabulated list of side effects, but it also notes that the frequency in clinical trials was actually similar in the drug and placebo groups[50]
.
Treatment options for chronic oedema
Chronic oedema occurs in a range of conditions including lymphoedema and venous insufficiency and is a common feature of patients with venous ulceration[69]
. It is important that treatment is tailored to the individual patient’s needs and comorbidities. The primary treatment for the majority of patients with chronic oedema is a combination of compression bandaging and exercise[26]
. However, drug therapy can have a place in treatment for some patients. The following are medicines that may be used.
Corticosteroids
This may seem counterintuitive, but there is a role for corticosteroids in the management of oedema in some patients with advanced cancer. Corticosteroid treatment is believed to reduce peritumour oedema and relieve compression of the venous and lymphatic systems, thereby reducing oedema[26]
. Dexamethasone may be given at a dose of 4mg–6mg twice daily for a one to two week trial and then stopped if ineffective or the dose reduced until the minimum effective dose is identified[26]
.
Diuretics
It is important to consider the cause of the chronic oedema when prescribing diuretic treatment. Diuretics are often ineffective, particularly in patients with lymphoedema,[26]
and can cause hypokalaemia, hyponatraemia and hypotension[15]
. Another concern is the rebound retention of sodium and water that occurs on stopping diuretic treatment[70]
. Diuretics are therefore not generally indicated in patients with lymphoedema, oedema caused by peripheral venous stasis, or oedema caused by CCBs[15]
. However, loop diuretics are indicated in the treatment of oedema associated with heart failure, which is commonly associated with peripheral oedema[71]
.
Not all patients fit neatly into one diagnosis — some will have both heart failure and lymphoedema. In these patients, a trial of diuretics might be appropriate. In some patients, such as those in whom corticosteroids are essential, diuretics can be helpful in countering the side effects of sodium and water retention[4]
.
Oxerutins
There has been interest in the use of benzopyrones, such as coumarin and oxerutins, in the treatment of chronic oedema because of lymphatic and venous disease[12]
. Coumarin is no longer available but oxerutins are licensed in the UK for the relief of symptoms of oedema associated with chronic venous insufficiency[72]
; however, it is considered less suitable for prescribing[15]
. In 2004, a Cochrane review of the use of benzopyrones in patients with lymphoedema found that it was not possible to draw conclusions about the effectiveness of benzopyrones used for the management of lymphoedema from the current available trials. Therefore, benzopyrones are not currently used in the routine management of lymphoedema[73]
.
Improving patient care
It is important that pharmacists and healthcare professionals work in line with the current guidance on polypharmacy, in particular being alert to the risk of adverse drug reactions[74]
, or adverse drug events[75]
. This will help identify medicines that may be causing or worsening a concurrent condition. When a patient who has developed oedema or one whose pre-existing oedema is worsening presents, the following points should be considered:
- Causal relationship — needs to be investigated;
- Possible dose reduction — may be sufficient to resolve the problem;
- Switching to an alternative — there might be a more suitable option for the patient;
- Withdrawal of the/the offending medicine — may be necessary.
Pharmacists and healthcare professionals should first consider whether this is a worsening of the patient’s condition or if it could be medicine related. If a patient has recently started a new medicine, further investigation may be required regarding the timing of this and the subsequent development or worsening of the oedema. The likelihood of that medicine causing oedema is also important and the SPC for the product may be more helpful than the British National Formulary (BNF) in establishing this.
If there is a clear relationship between the introduction of a medicine and the development or exacerbation of oedema, a dose reduction or withdrawal of the drug might need to be considered. In some instances, it may be possible to switch to another drug, either from a different class, or within the same class. For example, in a hypertensive patient who develops CCB-associated oedema, it might be ideal to change to another class of drug; however, if that is not an option, then a decrease in dose, or switching to another CCB could help resolve or reduce the problem.
Notably, one study suggests that the combination of a renin-angiotensin-aldosterone system blocking drug (ACE inhibitor, angiotensin receptor blocker or direct renin inhibitor) with a CCB might reduce the incidence of CCB-induced oedema. The authors of the study also note that ACE inhibitors may be more effective than angiotensin receptor blockers but further evidence is required to confirm this[76]
.
Pharmacists can also give advice on other treatments that can influence patients’ quality of life. These are described in the following sections.
Analgesia
Pain — most commonly described as aching, heaviness and tenderness — has been reported in 50% of patients with chronic oedema[3]
. Non-drug measures such as compression may be effective in relieving pain[26]
, but it can also occur because of the increased weight of a limb and association with cellulitis or venous thrombosis, which these patients are predisposed to develop. Careful consideration should be given to the choice of analgesic, especially as these patients are likely to take it long term. An analysis of the French national pharmacovigilance database has led to concern about the use of NSAIDs because its results suggest that NSAIDs increase the risk of necrotising fasciitis and dermal infection[27]
. However, Souyri et al. found it was not possible to say whether NSAIDs increase the risk of necrotising complications in all patients, but notes that their use may mask the symptoms and delay diagnosis[68]
.
The British Lymphology Society and Lymphoedema support network ‘Consensus document on the management of cellulitis in lymphoedema’ states that it is advisable to avoid NSAIDs during an acute attack of cellulitis, as they may be associated with complications such as necrotising fasciitis[77]
. Other medicines that should be used with caution include anticonvulsant drugs pregabalin and gabapentin, which are used in the management of neuropathic pain and are listed as commonly causing peripheral oedema. In one study, patients taking gabapentin showed a higher incidence of peripheral oedema in those aged over 65 years than in younger patients[31]
. However, this is probably true for patients taking placebo too and it is hard to make an informed decision without accessing the full data.
Skin care
Patients with chronic oedema are susceptible to skin problems, therefore adequate skin care is an extremely important part of the management of chronic oedema, because this helps maintain skin integrity and reduce the risk of infection[78]
. Deep folds of the skin that result from extensive swelling provide an ideal environment for the development of bacterial and fungal infection. Chronic inflammation of the skin tissues causes the deposition of fibrin and collagen, resulting in skin thickening[69]
. Fibrosclerotic tissue describes an affected area of skin in which inflammatory proteins of the lymph, including fibrin, have built up and caused the tissues of the skin to harden[69]
. Fibrosclerotic areas will remodel over time with appropriate compression therapy[69]
.
Skin should be cared for to improve and preserve the barrier function by washing with natural and pH neutral soaps. Skin folds should be kept clean and dry and moisturised with hypoallergenic emollients, which are used to re-establish the skin’s protective lipid layer and need to be applied frequently. Skin also needs to be monitored for any trauma that might be susceptible to infection. Patients should be taught good skin hygiene because if skin changes are left untreated they can quickly escalate in severity.
Infection
Fungal infections (e.g. tinea pedis) are common in oedematous weeping legs, especially between the toes[79]
. There is increasing evidence that tinea pedis infections may provide a portal of entry for bacteria and increase the risk of cellulitis[80]
. Cellulitis is an infection of connective tissue between neighbouring tissues and organs, commonly resulting from bacterial infection and can be located in the toes, dorsum and plantar surfaces of the feet and lower leg. It is generally reddish and painful[81]
.
Skin infections in patients with chronic oedema are primarily caused by Group A β-haemolytic streptococcal bacteria and, less commonly, staphylococcal bacteria[82]
,[83]
. Patient education should be aimed at prevention through meticulous skin hygiene and management of oedema and drainage. To prevent recurring fungal infections, patients should be encouraged to identify and treat symptoms early with a topical antifungal to avoid the risk of recurrent cellulitis. Where cellulitis does occur, or is suspected, an urgent referral is necessary as prompt antibiotic treatment is essential. Complications of cellulitis include fasciitis, myositis, subcutaneous abscesses, septicaemia, post-streptococcal nephritis and death[84]
.
Mental health
The physical changes associated with chronic oedema can have serious effects on mental wellbeing. Having to adapt clothing and footwear to disguise enlarged, disfigured and often exudating limb causes embarrassment and affects patients’ self-esteem and body image[2]
. Acute complications due to the condition cause major disruption to life, and the chronic nature of the condition significantly impairs quality of life, affecting the ability to function physically, socially and emotionally[3]
. Employment is often affected with (in one study) 80% of patients having to take time off, 2% having to change jobs because of their oedema, and 8% having to give up work because of it[3]
. Loss of control, fear, depression and social isolation result and pharmacists should be sensitive to these issues and prepared to signpost patients appropriately.
Importance of early diagnosis and referral
The importance of early diagnosis of chronic lower limb oedema cannot be overemphasised, to prevent early oedema becoming chronic. Pharmacists and other healthcare professionals should be encouraged to visually look for signs of chronic oedema when assessing patients and to refer patients where possible to a specialist local service. Tissue viability teams are starting to develop specific chronic oedema referral and treatment pathways which are helping to reduce the development of costly, long term complications.
Communication between the multidisciplinary team is essential to highlight common medicines that may cause or exacerbate chronic oedema as many healthcare professionals are unaware of this and may not realise that the expertise of the pharmacist (e.g. identifying medicines that are causing or contributing to the development of lower limb oedema and training regarding drug therapy in these patients) could help to control this condition.
Financial and conflicts of interest disclosure:
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Bianchi J, Vowden K & Whitaker J. Chronic oedema made easy. Wounds UK 2012;8(2):1–4. Available at: http://www.wounds-uk.com/pdf/content_10473.pdf (accessed June 2017)
[2] Todd M. Chronic oedema: impact and management. Br J Nurs 2013;22(11):623–627. doi: 10.12968/bjon.2013.22.11.623
[3] Moffat CJ, Franks PJ, Doherty DC et al. Lymphoedema: an underestimated health problem. Q J Med 2003;96(10):731–738. doi: 10.1093/qjmed/hcg126
[4] Keeley V. Drugs and lymphoedema: those which may cause oedema or make lymphoedema worse. Lymph Link 2012;24(4). Available at: http://www.lymphnet.org/membersOnly/dl/reprint/Vol_24/Vol_24-N4_Drugs_LE.pdf (accessed June 2017)
[5] Schweickert AJ & Arroyo JP. Back to basics in physiology. Elsevier Science 2013.
[6] Cho S & Atwood JE. Peripheral edema. Am J Med 2002;113:580–586. doi:10.1016/S0002-9343(02)01322-0
[7] Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol 1896;312–326. doi: 10.1113/jphysiol.1896.sp000596
[8] Mohrman DE & Heller LJ. Cardiovascular Physiology, 3rd Edition. McGraw-Hill International Editions, 1991.
[9] Levick JR & Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 2010;87:198–210. doi: 10.1093/cvr/cvq062
[10] Mortimer PS & Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 2014;124(3):915–921. doi: 10.1172/JCI71608
[11] Ely JW, Osheroff JA, Chambliss L et al. Approach to leg oedema of unclear etiology. J Am Board Fam Med 2006;19(2):148–160. doi: 10.3122/jabfm.19.2.148
[12] Keeley V. Drugs that may exacerbate and those used to treat lymphoedema. J Lymphoedema 2008;3(1):57–65. Available at: http://www.woundsinternational.com/media/issues/800/files/content_11122.pdf (accessed June 2017)
[13] Medicines and Healthcare products Regulatory Agency. Filgrastim and pegfilgrastim: risk of capillary leak syndrome. Drug Safety Update 2013. Available at: https://www.gov.uk/drug-safety-update/filgrastim-and-pegfilgrastim-risk-of-capillary-leak-syndrome (accessed June 2017)
[14] Telinius N, Mohanakumar S, Majgaard J et al. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 2014;592(21):4697–4714. doi: 10.1113/jphysiol.2014.276683
[15] Joint Formulary Committee, 2015. British National Formulary. 72. London: BMJ Publishing Group and Pharmaceutical Press.
[16] Topham EJ & Mortimer PS. Chronic lower limb oedema. Clin Med 2002;2:28–31. doi: 10.7861/clinmedicine.2-1-28
[17] Sica D. Calcium channel blocker-related peripheral edema: can it be resolved? J Clin Hypertens 2003;5(4):291–297. doi: 10.1111/j.1524-6175.2003.02402.x
[18] UK Medicines Information Q&A 322.3. What are the reported incidences of ankle oedema with different calcium channel blockers? November 2015. Available at: https://www.sps.nhs.uk/articles/what-are-the-reported-incidences-of-ankle-oedema-with-different-calcium-channel-blockers/ (accessed June 2017)
[19] Istin (Amlodipine). Pfizer Ltd. Summary of Product Characteristics, May 2015.
[20] Plendil (Felodipine). AstraZeneca UK Limited. Summary of Product Characteristics, November 2016.
[21] Motens (Lacidipine). GlaxoSmithKline UK. Summary of Product Characteristics, June 2016.
[22] Zanidip (Lercanidipine). Recordati Pharmaceuticals Limited. Summary of Product Characteristics, April 2006.
[23] Adalat (Nifedipine). Bayer plc. Summary of Product Characteristics, September 2016.
[24] Tildiem (Diltiazem). Sanofi. Summary of Product Characteristics, July 2016.
[25] Securon SR (Verapamil). Mylan Products Limited. Summary of Product Characteristics, November 2016.
[26] Keeley V. Pharmacological treatment for chronic oedema. Br J Community Nurs 2008;13(4):S4–S10. doi: 10.12968/bjcn.2008.13.Sup2.29394
[27] Necrotising fasciitis, dermal infections and NSAIDs: caution. Prescrire Int 2007;16(87):17. Available at: http://english.prescrire.org/en/SummaryDetail.aspx?IssueId=87 (accessed June 2017)
[28] Souyri C, Olivier P, Grolleau S et al. French Network of Pharmacovigilance Centres. Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol 2008;33(3):249–255. doi: 10.1111/j.1365-2230.2007.02652.x
[29] Cardura Tablet 2mg (Doxazosin mesilate). Pfizer Limited. Summary of Product Characteristics, February 2017.
[30] Detrusitol 1mg film-coated tablets (Tolterodine tartrate). Pfizer Limited. Summary of Product Characteristics, July 2015.
[31] Neurontin (Gabapentin). Pfizer Ltd. Summary of Product Characteristics, December 2016.
[32] Lyrica (Pregabalin). Pfizer Ltd. Summary of Product Characteristics, November 2016.
[33] Isocarboxazid tablets 10mg (Isocarboxazid). Alliance Pharmaceuticals. Summary of Product Characteristics, January 2015.
[34] Nardil tablets (Phenelzine sulphate). Kyowa Kirin Ltd. Summary of Product Characteristics, December 2016.
[35] Zispin SolTab (Mirtazapine). Merck, Sharp and Dohme Ltd. Summary of Product Characteristics, January 2017.
[36] Family Practice Notebook. medicine causes of oedema. Available at: http:// www.fpnotebook.com/renal/pharm/MdctnCsOfEdm.htm (accessed June 2017)
[37] Actos (Pioglitazone). Takeda UK Ltd. Summary of Product Characteristics, May 2016.
[38] Onglyza 2.5mg & 5mg film-coated tablets (Saxagliptin hydrochloride). AstraZeneca UK Limited. Summary of Product Characteristics, April 2016.
[39] Januvia 25mg, 50mg, 100mg film-coated tablets (Sitagliptin phosphate monohydrate). Merck Sharp & Dohme Limited. Summary of Product Characteristics, January 2016.
[40] Galvus 50mg Tablets (Vildagliptin). Novartis Pharmaceuticals UK Ltd. Summary of Product Characteristics, March 2017.
[41] Trobalt (Retigabine). GlaxoSmithKline UK. Summary of Product Characteristics, April 2016.
[42] Zonegran 25, 50, 100mg Hard Capsules (Zonisamide). Eisai Ltd. Summary of Product Characteristics, January 2017.
[43] Vfend (Voriconazole). Pfizer Ltd. Summary of Product Characteristics, January 2017.
[44] Hydralazine Tablets BP 25mg. Actavis UK Ltd. Summary of Product Characteristics, August 2016.
[45] Loniten (Minoxidil). Pfizer Ltd. Summary of Product Characteristics, June 2016.
[46] Priadel (Lithium). Aventis Pharma Limited. Summary of Product Characteristics, June 2015.
[47] Zyprexa (Olanzapine). Eli Lilly and Company Limited. Summary of Product Characteristics, February 2017.
[48] Seroquel (Quetiapine). AstraZeneca UK Limited. Summary of Product Characteristics, August 2016.
[49] Risperdal (Risperidone). Janssen-Cilag Ltd. Summary of Product Characteristics, June 2015.
[50] Fosamax (Alendronic acid). Merck Sharp and Dohme Ltd. Summary of Product Characteristics, June 2016.
[51] Actonel (Risedronate sodium). Warner Chilcott UK Limited. Summary of Product Characteristics, January 2016.
[52] Taxotere (Docetaxel trihydrate). Sanofi. Summary of Product Characteristics, May 2016.
[53] Alimta 100mg/500mg powder for concentrate for solution for infusion (Pemetrexed disodium). Eli Lilly and Company Limited. Summary of Product Characteristics, January 2017.
[54] Neupogen Singleject 30 MU (0.6mg/ml) (Filgrastim). Amgen Ltd. Summary of Product Characteristics, October 2015.
[55] Neulasta (Pegfilgrastim). Amgen Ltd. Summary of Product Characteristics, May 2015.
[56] Certican Tablets (Everolimus). Novartis Pharmaceuticals UK Ltd. Summary of Product Characteristics, December 2016.
[57] Rapamune (Sirolimus). Pfizer Limited. Summary of Product Characteristics, February 2017.
[58] Prograf 0.5mg, 1mg, 5mg Hard Capsules (Tacrolimus). Astellas Pharma Ltd. Summary of Product Characteristics, June 2015.
[59] Amantadine hydrochloride 100mg capsules (Symmetrel), (Amantadine hydrochloride). Auden Mckenzie (Pharma Division) Ltd. Summary of Product Characteristics, April 2016.
[60] Cabaser 1mg Tablets (Cabergoline). Pfizer Limited. Summary of Product Characteristics, January 2014.
[61] Mirapexin prolonged-release tablets (Pramipexole dihydrochloride monohydrate). Boehringer Ingelheim Limited. Summary of Product Characteristics, February 2017.
[62] Requip 5mg Tablets (Ropinirole hydrochloride). GlaxoSmithKline UK. Summary of Product Characteristics, September 2016.
[63] Neupro (Rotigotine). UCB Pharma Limited. Summary of Product Characteristics, January 2016.
[64] Nexium 20mg Tablets (Esomeprazole magnesium trihydrate). AstraZeneca UK Limited. Summary of Product Characteristics, January 2017.
[65] Losec Capsules 20mg (Omeprazole). AstraZeneca UK Limited. Summary of Product Characteristics, January 2017.
[66] Pantoloc Control 20mg gastro-resistant tablets (Pantoprazole). GlaxoSmithKline Consumer Healthcare. Summary of Product Characteristics, March 2017.
[67] Pariet (Rabeprazole). Eisai Ltd. Summary of Product Characteristics, March 2017.
[68] MHRA letter from Faiza Farooq. Query number GENQ-00117290. Received via email December 2016.
[69] Keast D. (2013) ‘Lymphoedema’. In Flanagan, M. (Ed). Wound Healing and Skin Integrity: principles and practice. Wiley Blackwell.
[70] Missouris CG, Cappuccio FP, Markandu ND et al. Diuretics and oedema: how to avoid rebound sodium retention. Lancet 1992;339(8808):1546. doi: 10.1016/0140-6736(92)91317-2
[71] Brater DC. Diuretic therapy. N Engl J Med 1998;339:387–395. doi: 10.1056/NEJM199808063390607
[72] Paroven capsules (Oxerutins). Novartis Consumer Health UK Ltd. Summary of Product Characteristics, March 2016.
[73] Badger CMA, Preston N, Seers K & Mortimer P. Benzopyrones for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev 2004;(2):CD003140. doi: 10.1002/14651858.CD003140.pub2
[74] NHS Scotland. Polypharmacy Guidance 2015. Available at: http://www.sign.ac.uk/pdf/polypharmacy_guidance.pdf (accessed June 2017)
[75] The Kings Fund. Polypharmacy and medicines optimisation 2013. Available at: http://www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf (accessed June 2017)
[76] Makani H, Bangalore S, Romero J et al. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral oedema. Am J Med 2011;124(2):128–135. doi:10.1016/j.amjmed.2010.08.007
[77] British Lymphology Society and Lymphoedema Support Network. Consensus Document on the Management of Cellulitis in Lymphoedema 2016. Available at: http://www.lymphoedema.org/index.php/cellulitis/cellulitis-in-lymphoedema (accessed June 2017)
[78] Vlahovi T & Schleicher S. Skin Disease of the Lower Extremities: A Photographic Guide. Malvern, PA: HMP Communication LLC (2012).
[79] Cabete J, Galhardas C, Apetato M et al. Onychomycosis in patients with chronic leg ulcer and toenail abnormalities. An Bras Dermatol 2015;90(1):136–139. doi: 10.1590/abd1806-4841.20152940
[80] Bristow IR & Spruce MC. Fungal foot infection, cellulitis and diabetes: a review. Diabet Med 2009;26(5):548–551. doi:10.1111/j.1464-5491.2009.02722.x
[81] Phoenix G, Das S & Joshi M. Diagnosis and management of cellulitis. BMJ 2012;345(7869):38–42. doi: 10.1136/bmj.e4955
[82] Gunderson CG & Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect 2012;64(2):148–155. doi: 10.1016/j.jinf.2011.11.004
[83] Jeng A, Beheshti M, Li J et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine 2010;89(4):217–226. doi: 10.1097/MD.0b013e3181e8d635
[84] Clinical Resource Efficiency Support Team (CREST). Guidelines on the management of cellulitis in adults, 2005 DHSS Northern Ireland.


