Dr Kari Lounatmaa / Science Photo Library
Clostridium difficile is a spore-producing Gram-positive anaerobic bacterium, and a common cause of healthcare-associated infection. C. difficile is an asymptomatic commensal in 2–3% of the adult population, but among some patients prescribed antimicrobials it is also a leading cause of antibiotic-associated diarrhoea and can lead to colitis[1]
.
Mandatory reporting of C. difficile infection (CDI) in England and Wales means that detailed epidemiological data are available. These data suggest that between 2004 and 2008, quarterly rates of CDI fluctuated from 10,000 to 17,000 cases[2]
. From 2008 to 2016, these rates have fallen year-on-year and are now around 3,000 cases per quarter (data from 2004 to 2007 include patients aged over 65 years, after which time all cases in patients aged two years and over are included)[2]
. This reduction in case rate has been largely attributed to changes in infection prevention and control and in antimicrobial stewardship. High rates of CDI have also been observed across Europe[3]
and the United States[4]
.
CDI has notable budgetary implications for healthcare providers. A systematic review published in 2012 identified costs of £4,577 per case in Ireland, £6,986 in the UK and £8,843 in Germany, but only £2,917 in Finland[5]
. Of greater concern are deaths associated with CDI – from 1999 to 2007 there was an eight-fold rise, peaking at more than 4,000 deaths per year in the UK[6]
(see figure 1).
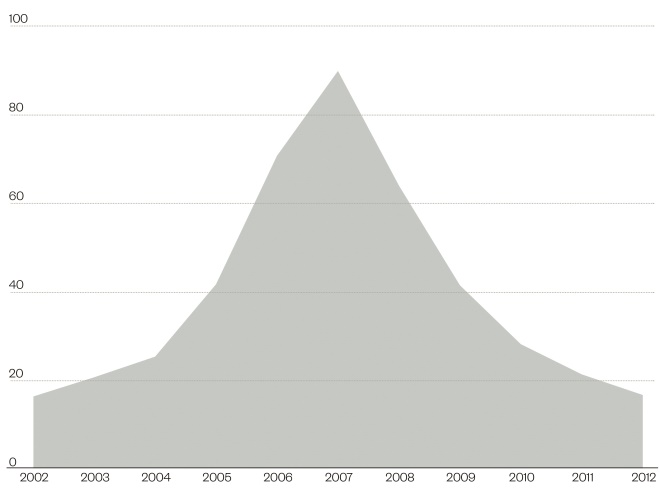
Figure 1: Mortality rates for deaths mentioning Clostridium difficile, England and Wales, deaths registered between 2002 and 2012
Source: Office of National Statistics
Age standardised rates per million population
In inpatient settings, several key risk factors can be identified for CDI (see ‘Box 1: Risk factors for CDI’). There has been a rise in community-associated CDI, albeit to a lesser degree than in inpatient settings, however, it is unclear whether this has arisen due to dissemination from inpatient settings[7]
, food sources[8]
or rising antacid prescription[9]
, all of which may be contributing factors. This article will discuss the microbiology, diagnosis and management of CDI, including novel and emerging treatments such as faecal microbiota transplant (FMT).
Microbiology
An anaerobic Gram-positive organism, two main virulence factors make C. difficile particularly pathogenic to humans. Virulence factors are molecules produced by pathogenic bacteria and other organisms that add to their effectiveness and enable them to achieve the following: colonisation of a niche in the host (this includes attachment to cells); immunoevasion, evasion of the host’s immune response; immunosuppression, inhibition of the host’s immune response; entry into and exit out of cells (if the pathogen is an intracellular one) and obtain nutrition from the host.
In the case of C. difficile, it is able to sporulate, and these spores can remain viable on numerous types of surface and material for prolonged periods, and can resist being killed by a variety of cleaning products[10]
. C. difficile can, therefore, spread quickly through and between healthcare facilities by patients, staff and on fomites (e.g. clothes, utensils and furniture – see previous article on healthcare-associated infection). Once ingested and after passage through the low pH gastric environment, these spores germinate and C. difficile establishes bowel colonisation. Infection (rather than colonisation) with C. difficile is linked to two exo-proteins: toxin A (TcdA) and toxin B (TcdB)[11]
,[12]
.
Diagnosis
As C. difficile can be both a commensal and an infecting pathogen, and as the available tests have sub-optimal sensitivity and specificity, no single test can confirm or refute a diagnosis of CDI. Patients should, therefore, only be tested for CDI when they actually have diarrhoea and an infective aetiology is suspected[13]
. Three main tests are available:
1. Glutamate dehydrogenase (GDH – a C. difficile antigen) is often used as a screening test, but lacks specificity;
2. Toxin A/B gene polymerase chain reaction (PCR) is sensitive, but detection of the toxin genes does not equate to expression/production of the toxin;
3. Toxin A/B detection, which has variable sensitivity, but when present provides strong evidence for CDI in the context of diarrhoea.
A two-stage testing algorithm is widely advocated in many countries, including in the UK[13]
. Both GDH and toxin A/B detection are used; a positive result from both tests has a 91.4% positive predictive value, while both tests being negative has a 98.9% negative predictive value[14]
.
For investigation of C. difficile outbreaks, ribotyping (a molecular technique for bacterial identification that uses information from rRNA-based phylogenetic analyses) is currently the main mechanism to compare strains. It is likely that in due course whole genome sequencing will become more accessible and potentially provide a finer resolution typing modality[15]
.
Management
A multidisciplinary team approach is indicated for the management of CDI, incorporating microbiologists or infectious diseases physicians, antimicrobial pharmacists and infection control nurses, and they should have access to the expertise of gastroenterologists and gastro-intestinal surgeons[16]
.
Assessment of patients diagnosed with CDI is based upon review and intervention of factors contributing to the infection (i.e. review of concomitant antimicrobial therapy, antacid use), interventions to prevent onward spread (i.e. isolation in a side room with dedicated en-suite bathroom and apron/glove/hand washing facilities with soap for visitors and staff), and completion of a severity assessment[17]
. The latter involves clinical examination and assessment of stool frequency and consistency, correlated with inflammatory markers and, where performed, abdominal imaging and endoscopy findings.
While several severity scoring systems exist, Public Health England (PHE), an executive agency of the Department of Health in the UK, guidance[18]
suggests the presence of any one of the following is suggestive of severe disease:
- Severe colitis (either from clinical abdominal signs or from radiological investigations);
- White cell count >15 x 109/L;
- Acutely rising creatinine (e.g. >50% increase above baseline);
- Temperature >38.5°C.
Management of CDI depends on this severity assessment. After stopping concomitant systemic antimicrobials and antacids where possible, and after ensuring adequate fluid balance, targeted C. difficile antimicrobial therapy is the mainstay of treatment (see section on treatment). For patients who deteriorate despite optimal medical therapy, a surgical opinion should be sought, together with consideration given to using intravenous immunoglobulin[18]
.
Box 1: Risk factors for Clostridium difficile infection (CDI)
- Age: CDI rates in patients 65 years and older are up to ten-fold higher than that for younger patients.
- Antimicrobial choice: almost all antibiotics predispose to CDI, although some have a well-defined impact on the odds ratio (OR) for developing CDI[19]
:- cephalosporins (OR: 3.84–26);
- clindamycin (OR: 2.12–42);
- penicillin-inhibitor compounds (e.g. co-amoxiclav) (pooled OR: 22.1; 6.5–75.4);
- quinolones (pooled OR: 8; 4.5–14.3).
- Antimicrobial duration: <4 days experience significantly (p=0.009) fewer episodes of C. difficile -associated disease (CDAD) compared with those who are treated for longer[20]
. - Acid-suppressing medications:
- H2 receptor antagonists increases risk by 53% (95% CI, OR:1.2–2.10);
- daily proton pump inhibitor therapy increases the risk by 74% (95% CI, 1.39–2.18).
- Length of stay: risk increasing after seven days of hospitalisation.
- Recent hospitalisation: within the last two months.
- Location: admission to a room where the previous patient had C. difficile
[21]
.
More information: Healthcare-associated infections has been covered in more detail in a previous learning article
Treatment
For many years, metronidazole and vancomycin represented the only pharmacological treatment options for CDI. Metronidazole has high oral bioavailability, associated with reliably therapeutic colonic luminal exposure[22]
. Conversely, vancomycin has extremely limited oral bioavailability, whereas the intravenous (IV) preparation has minimal penetration into gut mucosa[23]
.
Metronidazole
Oral metronidazole has been demonstrated in randomised controlled trials (RCTs) to be non-inferior to vancomycin for the treatment of non-severe CDI[24]
. Concern has arisen regarding increased spread of vancomycin-resistant enterococci (VRE) if oral vancomycin were used as first-line therapy[25]
. There is considerable cost disparity between oral metronidazole and vancomycin [UK price of a standard treatment course ≈ £3 vs £260, respectively][26]
. Metronidazole remains first-line treatment for non-severe CDI[18]
. Metronidazole liquid contains the pro-drug metronidazole benzoate, which requires activation via gastric enzymes. In patients with diarrhoea, there is a theoretical risk that efficacy may be compromised because of reduced exposure to gastric enzymes. Therefore, metronidazole tablets may be crushed and dispersed in water for administration to patients with swallowing difficulties or nasogastric tubes in preference to using the liquid preparation.
Vancomycin
Oral vancomycin remains the first-line treatment for severe CDI. A dose of 125mg six-hourly is recommended by PHE, Infectious Disease Society of America and the European Society of Clinical Microbiology and Infectious Diseases[27]
; however, the superiority of higher doses has not been demonstrated to date[28]
.
Recent data suggest combination therapy (IV metronidazole plus oral vancomycin) may confer an additional survival benefit in the region of 20% for critically unwell patients over vancomycin monotherapy[29]
.
Vancomycin capsules are not suitable for use via a nasogastric tube. Instead, vancomycin injection can be reconstituted and diluted in 30ml of water for administration via a nasogastric tube. Reconstituted and diluted vancomycin injection can also be administered orally in patients with swallowing difficulties (for more information on how to tailor medications for patients with swallowing difficulties, see this previous learning article).
Fidaxomicin
The novel macrocyclic antibiotic fidaxomicin has a narrow spectrum of activity. Data from the phase III RCTs, OPT-80-003 and OPT-80-004 suggest that fidaxomicin is non-inferior to vancomycin for the treatment of non-life-threatening CDI[30]
,[31]
. Moreover, fidaxomicin was associated with a significantly reduced risk of recurrent infection in the OPT-80-003 study (15.4% vs 25.3%, p=0.004)[30]
. Superior efficacy (in cure rate and disease-recurrence end points, respectively) of fidaxomicin versus vancomycin in patients with cancer and in patients receiving concomitant antimicrobials was demonstrated. No difference in efficacy in the treatment of hyper-virulent CDI caused by strains of ribotype 027 was found[31]
,[32]
.
Fidaxomicin is around seven times the cost of oral vancomycin or 600 times the cost of metronidazole for a standard 10-day course[26]
. However, detailed cost-effectiveness analyses, modelling various healthcare settings, suggest that fidaxomicin is cost-effective in severe disease and, particularly, for patients with high risk of recurrence[33]
. This is attributed to reduced recurrence rates and decrease in spread of C. difficile spores. PHE recommend that fidaxomicin should be used at a dose of 200mg BD for 10 days in recurrent CDI and considered in patients with severe CDI who are thought to be at high risk of recurrent infection[18]
. This includes elderly patients with multiple co-morbidities who are prescribed concomitant antibiotics[18]
.
Duration of therapy
The recommended duration of therapy for CDI is 10–14 days and should be guided by clinical response. The licensed duration of fidaxomicin is 10 days.
Probiotics and other treatment options
Probiotics
Antimicrobial therapy reduces diversity within the normal gut microbiome, a state felt to be central to the pathogenesis of CDI[34]
. Probiotics are microorganisms that colonise the gut following ingestion, and thus confer putative health benefits.
Probiotics have an uncertain role in the prevention and treatment of CDI. Although a meta-analysis of RCTs suggests that probiotics conferred significant benefit in preventing CDI[35]
, the PLACIDE study showed no benefit[36]
. A study involving Saccharomyces boulardii (a probiotic yeast), found no interventional benefit[37]
. Based on current data, it is unclear whether certain patient populations derive more benefit from adjunctive probiotic therapy than others. Such is the relative diversity of available probiotics that it is unclear whether choice of organism has an impact on outcomes. Some probiotic organisms, which are non-pathogenic in the normal host, may cause atypical disease presentations in the immunosuppressed patient[38]
.
Other treatment options
Intracolonic vancomycin may be considered in severe disease[18]
. Intravenous immunoglobulin (IVIG) at a dose of 400mg/kg stat may be considered as salvage therapy in severe CDI[18]
. The rationale for use is that IVIG is thought to bind to and neutralise toxin A. Teicoplanin has been used in the treatment of refractory CDI[39]
but evidence is limited and oral administration is complicated by having to give the injection orally.
There are a number of novel therapies in the pipeline. Surotomycin, a selective bactericidal cyclic lipopeptide, has recently been demonstrated in a phase II RCT to produce similar cure rates but lower recurrence rates compared with oral vancomycin[40]
. Cadazolid, a novel fluoroquinolone-oxazolidinone, was associated with reduced recurrence rates compared with oral vancomycin in a phase II RCT[41]
. Bezlotoxumab, a monoclonal antibody targeting CDI toxin B, was approved by the US Food and Drug Administration (FDA) in October 2016 to reduce recurrence of CDI[42]
. As it is not an antibiotic, bezlotoxumab should only be used in combination with appropriate antibiotics for the treatment of CDI.
Other treatment options currently being investigated include rifaximin[43]
and vaccines[44]
.
For recurrent CDI, PHE guidance recommends that FMT may be considered[18]
.
Faecal microbiota transplant
Like probiotics, FMT aims to restore the gut microbiome and function, although the exact mechanism is not clearly understood. FMT was first documented for use in pseudomembranous colitis in 1958[45]
, but it was not widely adopted until recently. The National Institute for Health and Care Excellence (NICE), England’s health technology assessment body, approval was granted in 2014[46]
and FMT solution has been classified as a medicinal product.
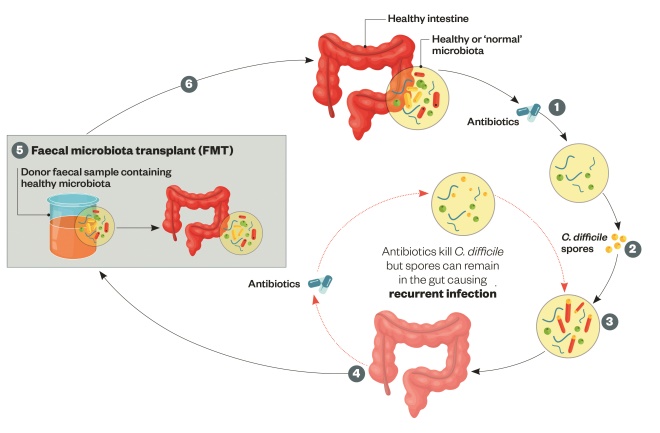
Figure 2: C. difficile infection and the role of faecal microbiota transplantation
Faecal microbiota transplant (FMT) aims to restore the gut microbiome and function, although the exact mechanism is unclear. Current Public Health England guidance recommends FMT should be considered in recurrent C. difficile infection (CDI).
1) Ingesting antibiotics results in a reduction of microbial species and diversity.
2) Spores picked up from the environment are ingested.
3) The C. difficile spores germinate resulting in dysbiosis of the gut microbiota.
4) This can result in the development of CDI (characterised by severe diarrhoea, abdominal pain, nausea and fever). Inflammation and cell death occurs due to presence of toxins. Severe CDI can cause pseudomembranous colitis.
5) Faecal microbiota transplant (FMT): The faecal sample is filtered and administered by enema, transcolonic infusion or nasoduodenal infusion.
6) The result is restoration of stable, healthy microbiota.
Current PHE guidance recommends that FMT may be considered in recurrent CDI[18]
. The European Society of Clinical Microbiology and Infectious Diseases recommends FMT, in combination with oral vancomycin, for the treatment of multiple recurrent CDI refractory to antibiotic therapy[47]
.
Protocol
There is no consensus on the optimum protocol for FMT. Following patient consent, FMT is generally co-ordinated by the gastroenterology team.
A stool must come from a healthy donor following comprehensive screening; traditionally a family member, but now anonymous donors are used. A questionnaire is used to assess the donor’s medical history and risk for infectious diseases. Blood and stool are screened to test for viruses, parasites or enteric pathogens that could potentially be transmitted to the FMT recipient[46]
. Donor screening is costly[48]
but a cost–effectiveness analysis performed in a US setting identified FMT by colonoscopy to be a cost-effective treatment for recurrent CDI[49]
.
Fresh or frozen manipulated donor faeces are administered as a solution via enema, nasogastric tube or colonoscopy. The donor stool is mixed with water, normal saline, yoghurt or milk[46]
to produce a solution with minimal odour. Recently faecal material administered via capsules has shown promise[50]
and is likely to be a more aesthetically acceptable route of FMT.
Antimicrobial therapy is stopped 24–48 hours prior to FMT to increase the retention of transplanted stool. A bowel preparation is used prior to the FMT procedure to reduce C. difficile load in the intestine. If nasogastric tube or upper endoscopic FMT administration is being considered, a proton pump inhibitor (PPI) may be used the evening before and on the morning of FMT to decrease gastric acid.
Results from published studies
Diarrhoea generally resolves within 48–72 hours of FMT. A systematic review found symptoms resolved in 89% of patients with recurrent CDIs after a single FMT via a nasoduodenal tube with a 4% relapse rate[51]
. Similar cure rates were reported from an open-label RCT; diarrhoea resolved in 81% of patients treated with FMT compared with 31% treated with vancomycin alone and 23% treated with vancomycin and a bowel lavage[52]
. Of patients in whom an initial FMT, 66% had resolution of diarrhoea after a second transplant[52]
.
A small feasibility study using capsules containing filtered, diluted and frozen stool from healthy donors also showed promising results. Symptoms resolved in 70% of patients with no recurrence within eight weeks. Around 67% of patients with a failed initial FMT achieved resolution of diarrhoea following a second transplant[53]
.
The efficacy and safety of FMT in primary or severe CDI has not been established, with current evidence limited to case reports[54]
,[55]
. There is concern about withdrawing antibiotics for the treatment of CDI in severe disease[56]
and use of FMT in patients with fulminant disease may delay surgical intervention.
Adverse events
The most common adverse events reported from the first RCT were diarrhoea, belching and cramping, generally subsiding within three hours. Constipation was noted in 19% of patients at follow-up[53]
. Risks associated with nasogastric tubes and colonoscopy must be considered. Despite stringent screening criteria, there remains concern about the potential for transmission of infectious diseases from the donor to the recipient.
The long-term safety of FMT has not been established. The gut microbiome may be associated with conditions such as diabetes mellitus, colon cancer and obesity[57]
. Significant weight gain in a patient following FMT from an obese donor has been reported[58]
. Extended follow-up is needed to establish the long-term safety of FMT.
Delivery and feasibility
FMT requires a multi-disciplinary team approach involving infectious diseases doctors (microbiology or infectious diseases clinicians), gastroenterologists and pharmacists (ideally with a specialist interest in infection). Pharmacists can be involved as part of the team, especially in identifying eligible patients, screening potential donors through undertaking a comprehensive medical history, monitoring stool charts for clinical response and developing local FMT protocols. If FMT capsules become commercially available in the future, pharmacists and healthcare professionals working in pharmacy may be involved in their manufacture and supply.
Financial and conflicts of interest disclosure:
Orla Geoghegan has previously received educational grants from Astellas and Luke SP Moore has previously consulted for bioMérieux and DNA electronics and has received an educational grant from Eumedica. Mark Gilchrist has previously received educational grants from Astellas, Pfizer, Sanofi and MSD. Christopher Eades has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Moore LSP, Cooley N & Gilchrist M. Clostridium difficile: microbiology & infection. Clin Pharm 2012;4:250-254. Available at: http://www.pharmaceutical-journal.com/learning/learning-article/clostridium-difficile-microbiology-and-infection/11107951.article (accessed February 2017)
[2] Public Health England, 2016. Quarterly Epidemiological Commentary: Mandatory MRSA, MSSA and E. coli bacteraemia, and C. difficile infection data (up to October–December 2015). Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/506294/QEC_March_2016_final.pdf (accessed February 2017)
[3] Bauer MP, Notermans DW, van Benthem BH et al. ECDIS study group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011;377(9759):63-73. doi: 10.1016/S0140-6736(10)61266-4
[4] Kelly CP, LaMont JT. Clostridium difficile– more difficult than ever. N Engl J Med 2008;359(18):1932-1940. doi: 10.1056/NEJMra0707500
[5] Wiegand PN, Nathwani D, Wilcox MH et al. Clinical and economic burden of Clostridium difficile in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012;81(1):1-14. doi: 10.1016/j.jhin.2012.02.004
[6] Office for national statistics statistical bulletin. Deaths involving C. difficile: England and Wales 2006-2010. Published 23 August 2011. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingclostridiumdifficileenglandandwales/previousReleases%20 (accessed February 2017)
[7] Bauer MP, Veenedaal D, Verhoef L et al. Clinical and microbiological characteristics of community-onset Clostridium difficile infection in the Netherlands. Clin Microbiol Infect 2009;15(12):1087-1092. doi: 10.1111/j.1469-0691.2009.02853.x
[8] Pasquale V, Romano V, Rupnik M et al. Occurrence of toxigenic Clostridium difficile in edible bivalve molluscs. Food Microbiol 2012;31(2):309-312. doi: 10.1016/j.fm.2012.03.001
[9] Kuntz JL, Chrischilles EA, Pendergast JF et al. Incidence of and risk factors for community-acquired Clostridium difficile infection: a nested case-control study. BMC Infect Dis 2011;11:194. doi: 10.1186/1471-2334-11-194
[10] Dancer SJ.. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 2014;27(4):665-690. doi: 10.1128/CMR.00020-14
[11] Johnson S. Clostridium difficile toxins and severe C. difficile infection. J Infect Dis 2012;205(3):353-354. doi: 10.1093/infdis/jir752
[12] Kuehne SA, Cartman ST & Minton NP. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes 2011;2(4):252-255. doi: 10.4161/gmic.2.4.16109
[13] Clostridium difficile: updated guidance on diagnosis and reporting (6 March 2012). Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215135/dh_133016.pdf (accessed February 2017)
[14] Department of Health. Best Practice Guideline: Updated guidance on the diagnosis and report of Clostridium Difficile. Published 2012. Available at: https://www.gov.uk/government/publications/updated-guidance-on-the-diagnosis-and-reporting-of-clostridium-difficile (accessed February 2017)
[15] Eyre DW, Cule ML, Wilson DJ et al. Diverse Sources of C. difficile infection on whole genome sequencing. N Eng J Med 369:1195-1205. doi: 10.1056/NEJMoa1216064
[16] Hatton K, Cooley N, Demertzi E et al. The impact of a multidisciplinary clinical review of inpatients with Clostridium difficile infection. J Infect 2011;62(6):e82-e83. doi: 10.1016/j.jinf.2011.04.143
[17] Gilchrist M, Cooley N & Moore LSP. Clostridium difficile: managing infections. Clin Pharm 2012;4:257-261. Available at: http://www.pharmaceutical-journal.com/learning/learning-article/clostridium-difficile-managing-infections/11108015.article (accessed February 2017)
[18] Public Health England (PHE). Updated guidance on the management and treatment of Clostridium difficile infection. 2013. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/321891/Clostridium_difficile_management_and_treatment.pdf%20 (accessed February 2017)
[19] Stevens V, Dumyati G, Fine LS et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile Infection. Clin Infect Dis 2011;53(1):42-48. doi: 10.1093/cid/cir301
[20] Wistrim J, Norrby SR, Myhre EB et al. Frequency of antibiotic associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicobial Chemother 2001;47:43-50. doi: 10.1093/jac/47.1.43
[21] Freedberg DE, Salmasian H & Cohen B. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016. doi: 10.1001/jamainternmed.2016.6193
[22] Kling PA & Burman LG. Serum and tissue pharmacokinetics of intravenous metronidazole in surgical patients. Acta Chir Scand. 1989;155(6-7):347-350.
[23] Rao S, Kupfer Y, Pagala M et al. Systemic absorption of oral vancomycin in patients withClostridium difficile infection. Scand J Infect Dis 2011;43(5):386-388. doi: 10.3109/00365548.2010.544671
[24] Zar FA, Bakkanagari SR, Moorthi K et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45(3):302-307. doi: 10.1086/519265
[25] Fujitani S, George WL, Morgan MA et al. Implications for vancomycin-resistant Enterococcus colonization associated with Clostridium difficile infections. Am J Infect Control 2011;39(3):188-193. doi: 10.1016/j.ajic.2010.10.024
[26] British National Formulary (BNF) 72. London, UK: Pharmaceutical Press; 2016.
[27] Cohen SH, Gerding DN, Johnson S et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Infect Control Hosp Epidemiol 2010;31(5):431-455. doi: 10.1086/651706
[28] Lam SW, Bass SN, Neuner EAet al. Effect of vancomycin dose on treatment outcomes in severe Clostridium difficile infection. Int J Antimicrob Agents. 2013;42(6):553-558. doi: 10.1016/j.ijantimicag.2013.08.013
[29] Rokas KE, Johnson JW, Beardsley JR et al. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis 2015:civ409. doi: 10.1093/cid/civ409
[30] Louie TJ, Miller MA, Mullane KM et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. NEJM 2011;364(5):422-431. doi: 10.1056/NEJMoa0910812
[31] Cornely OA, Crook DW, Esposito R et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12(4):281-289. doi: 10.1016/S1473-3099(11)70374-7
[32] Cornely OA, Miller MA, Fantin B et al. Resolution of Clostridium difficile -associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol 2013;31(19):2493-2499. doi: 10.1200/JCO.2012.45.5899
[33] Nathwani D, Cornely OA, Van Engen AK et al. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother 2014;69(11):2901-2912. doi: 10.1093/jac/dku257
[34] Chang JY, Antonopoulos DA, Kalra A et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile- associated diarrhea. J Infect Dis 2008;197(3):435-438. doi: 10.1086/525047
[35] Rea MC, Clayton E, O’Connor PM et al. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol 2007;56(7):940-946. doi: 10.1099/jmm.0.47085-0
[36] Allen SJ, Wareham K, Wang D et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013;382(9900):1249-1257. doi: 10.1016/S0140-6736(13)61218-0
[37] Ehrhardt S, Guo N, Hinz R et al. Saccharomyces boulardii to prevent antibiotic-associated diarrhea: a randomized, double-masked, placebo-controlled Trial. Open Forum Infect Dis 2016: Oxford University Press. doi: 10.1093/ofid/ofw011
[38] Walker DK & Paez AP. Aortic valve endocarditis due to lactobacillus casei complicated by stroke and vertebral osteomyelitis: a case report. Infect Dis Clin Pract 2013;21(1):66-68. doi: 10.1097/IPC.0b013e318260206b
[39] Popovic N, Korac M, Nesic Z et al. Oral teicoplanin for successful treatment of severe refractory Clostridium difficile infection. J Infect Dev Ctries 2015;9(10):1062-1067. doi: 10.3855/jidc.6335
[40] Lee CH, Patino H, Stevens C et al. Surotomycin versus vancomycin for Clostridium difficile infection: Phase 2, randomized, controlled, double-blind, non-inferiority, multicentre trial. J Antimicrob Chemother. 2016;71(10):2964-2971. doi: 10.1093/jac/dkw246
[41] Louie T, Nord CE, Talbot GH et al. Multicenter, double-blind, randomized, phase 2 study evaluating the novel antibiotic cadazolid in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59(10):6266-6273. doi: 10.1128/AAC.00504-15
[42] Centre Watch Drug Information: Zinplava (bezlotoxumab). Available at: http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/100173/zinplava-bezlotoxumab (accessed February 2017)
[43] Rubin DT, Sohi S, Glathar M et al. Rifaximin is effective for the treatment of Clostridium difficile -associated diarrhea: results of an open-label pilot study. Gastroenterol Res Pract 2011;2011:106978. doi: 10.1155/2011/106978
[44] Sougioultzis S, Kyne L, Drudy D et al. Clostridium difficile toxoid vaccine in recurrent C. difficile -associated diarrhea. Gastroenterology 2005;128(3):764-770. doi: 10.1053/j.gastro.2004.11.004
[45] Eiseman B, Silen W, Bascom GS et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958;44: 854–859.
[46] National Institute for Health and Care Excellence (NICE). Faecal microbiota transplant for recurrent Clostridium difficile infection. 2014. Interventional procedures guidance [IPG485]. Available at: https://www.nice.org.uk/guidance/ipg485 (accessed February 2017)
[47] Debast SB, Bauer MP & Kuijper EJ, on behalf of the Committee. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014;20(s2):1–26. doi: 10.1111/1469-0691.12418
[48] Mullish B & Williams H. Obstacles to establishing an NHS faecal transplant programme. BMJ 2015;351. doi: 10.1136/bmj.h6043
[49] Konijeti GG, Sauk J, Shrime MG et al. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis.Clin Infect Dis 2014;58(11):1507-1514. doi: 10.1371/journal.pone.0149521
[50] Hirsch BE, Saraiya N, Poeth K et al. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis 2015;15:191. doi: 10.1186/s12879-015-0930-z
[51] Gough E, Shaikh H & Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infec Dis 2011;53(10):994–1002. doi: 10.1093/cid/cir632
[52] van Nood E, Vrieze A, Nieuwdorp M et al. Duodenal Infusion of donor feces for recurrent Clostridium difficile. NEJM 2013;368(5):407-415. doi: 10.1056/NEJMoa1205037
[53] Youngster I, Russell GH, Pindar C et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014;312(17):1772-1778. doi: 10.1001/jama.2014.13875
[54] You DM, Franzos MA & Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med 2008;148(8):632-633. doi: 10.7326/0003-4819-148-8-200804150-00024
[55] Trubiano JA, Gardiner B, Kwong JC et al. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol 2013;25(2):255. doi: 10.1097/MEG.0b013e32835b2da9
[56] Solari PR, Fairchild PG, Noa LJ et al. Tempered enthusiasm for fecal transplant. Clin Infect Dis 2014;59(2):319. doi: 10.1093/cid/ciu278
[57] Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease.Physiol Rev 2010;90(3):859-904. doi: 10.1152/physrev.00045.2009
[58] Alang N & Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2015;4;2(1):ofv004. doi: 10.1093/ofid/ofv004


