dpa picture alliance / Alamy
Summary
Dialysis involves passing the patient’s blood against a semi-permeable membrane, with dialysis solution on the other side. It is usually started in patients with stage 5 chronic kidney disease. The main options are haemodialysis (HD), which is performed several times a week using a dialysis machine, and peritoneal dialysis (PD), which uses the patient’s peritoneum. Around 73% of patients in the UK starting renal replacement therapy use HD, around 19.5% use PD, and 7.4% of patients have a kidney transplant without starting dialysis.
HD and PD differ significantly in terms of risks and benefits to the patient. There are also additional requirements that need to be considered, for example whether the patient can attend an outpatient clinic and whether it is possible to create venous access via an arteriovenous fistula for patients receiving HD.
The development of renal replacement therapy (RRT) provides patients with a viable way of carrying out the role of the kidney when they have established renal failure. It was first used successfully in 1924, and became part of the management plan for patients with end-stage renal disease in the 1960s.
The main types of RRT for established renal failure are[1]
:
- Haemodialysis (HD)
- Haemodiafiltration (HDF)
- Peritoneal dialysis (PD)
- Kidney transplantation
A kidney transplant is the preferred management option for patients with established renal failure. A review of the point prevalence of RRT found transplantation is the most common intervention (50% of patients), followed by HD and HDF (43% of cases) and PD in (7% of cases). HDF numbers are included within the HD prevalence due to lack of clarity of reporting from UK centres[2]
.
Not every patient is suitable for or wants to have a transplant, and donors may not be available. In these cases, dialysis becomes the treatment of choice, either for long-term management or until a suitable donor is found.
The incidence of patients starting dialysis in the UK is 108 per million population per year. This has remained constant since 2006 to 2012 (the latest year with data available), although improved survival rates means the number of patients receiving dialysis has increased. In 2012, 54,824 people were receiving dialysis[1]
, and the median age for starting dialysis was 64.6 years[2]
.
Currently, 73% of patients in the UK starting RRT begin with HD, 19.5% with PD and 7.4% of patients have a kidney transplant without starting dialysis[2]
. Age is an important factor in determining whether to have a transplant or receive dialysis.
There can be a survival benefit in selected elderly patients of having a transplant; however, these patients are at a higher risk of morbidity and mortality following transplant than they would be by using dialysis[3]
.
When is dialysis considered?
Dialysis is usually started in patients with chronic kidney disease (CKD) stage 5 with an estimated glomerular filtration rate (eGFR) of less than 15ml/min/1.73m2 and symptoms of uraemia (e.g. nausea, vomiting, weight loss, pain, acidosis, hyperkalaemia)[3]
. The average eGFR reading when dialysis is started in the UK is 8.5ml/min/1.73m2 (ref. 2).
The threshold for starting dialysis also depends on other co-morbidities. For example, patients with chronic kidney disease and diabetes often develop uraemia with symptoms presenting earlier, leading to a higher threshold for starting dialysis. Other parameters also need to be taken into account, including the patient’s serum phosphate and bicarbonate levels and if pharmacological intervention is not sufficient.
Dialysis needs to be considered earlier in patients who are malnourished despite treatment, on account of there being a higher mortality rate among patients who start dialysis in a malnourished state because of uremic symptoms.
Patients with CKD who develop associated pulmonary oedema that is not responsive to treatment also require dialysis[3],[4]
.
Principles of dialysis
Dialysis involves passing the patient’s blood against a semi-permeable membrane, with dialysis solution on the other side. At this stage, three processes can occur to remove unwanted waste products.
Passive diffusion occurs when a high to low concentration gradient is present between the patient’s blood and dialysis solution (dialysate) used. Waste products in the blood diffuse into the dialysate solution while essential minerals diffuse into the blood; diffusion stops once equilibrium is achieved. The size of the molecule determines whether diffusion occurs: only low molecular weight solutes and water are able to pass through the semi-permeable membrane and therefore red blood cells are not lost[1],[5],[6]
.
Ultrafiltration ensures excess fluid is cleared from the body through the use of a positive (blood) or negative (dialysate) pressure gradient, moving fluid from a high to low pressure region[1],[6]
.
Convection allows effective clearing of larger molecules from the blood by creating a higher hydrostatic pressure in the blood (using a blood pump), leading to the passive movement of solutes dissolved in fluid. Convection relates to solutes in fluids crossing the membrane where ultrafiltration is related to movement of fluid under pressure[1],[5]
.
HD uses diffusion and ultrafiltration, while HDF and PD use all three processes. HDF is outside the scope of this article.
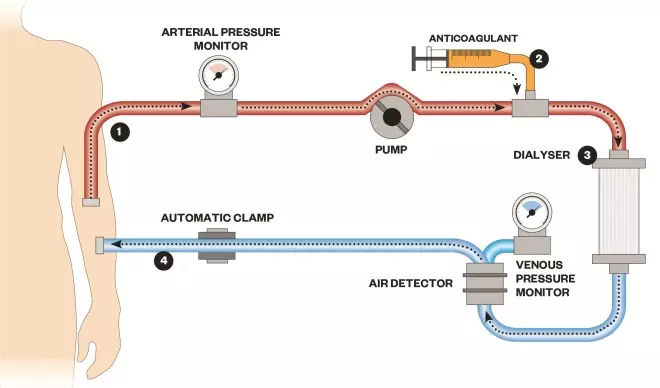
How a dialysis machine works
1) Blood is pumped out of a patient, often via an arteriovenous fistula
2) An anticoagulant is added to prevent the blood from clotting
3) Blood flows past the dialysate separated by a semi-permeable membrane
4) Blood is pumped back into the body
Haemodialysis
The main function of haemodialysis is to reduce the volume of uremic toxins in the blood, especially small and medium sized molecules, through diffusion. It also decreases the patient’s fluid volume via ultrafiltration and manages metabolic disturbances (e.g. maintaining the acid-base balance)[5]
.
This is achieved by a dialysis machine, which takes blood from the patient and pumps it towards the semi-permeable membrane. The dialysate is pumped from the opposite direction (countercurrent flow) to create a larger concentration gradient (see figure 1).
The dialysate is made up of electrolytes and water. A number of different dialysates are available to use with varying levels of electrolytes depending on the patient’s requirements.
Without dialysis, lack of kidney function will result in metabolic acidosis as the body fails to excrete excess acid and uses its serum bicarbonate to neutralise the pH. Therefore the dialysate needs to have a high bicarbonate concentration, which ensures a concentration gradient is present to allow bicarbonate to pass into the blood. Dialysis aims to move the patient from a state of mild metabolic acidosis to mild metabolic alkalosis, which prevents the acidosis from getting too severe between dialysis sessions.
Potassium needs to be removed from the blood. However, a small concentration of potassium is included in dialysates to prevent potassium being removed too quickly, minimising the risk of cardiac arrhythmias or cardiac death. The aim of potassium management during dialysis is to treat hyperkalaemia but not result in a significant hypokalaemia[5]
.
Glucose may also form part of the dialysate, and is particularly useful for patients with diabetes to prevent hypoglycaemia during therapy[6]
.
An anticoagulant is usually used to maintain patency and prevent clots within the extracorporeal circuit used in HD, which may lead to the dialyser becoming blocked[6]
. It is administered through the extracorporeal circuit; the most commonly used anticoagulant is unfractionated heparin, although a number of renal units now use a low molecular weight heparin instead. If these cannot be used (e.g. the patient is at increased risk of bleeding), a continuous infusion of epoprostenol may be used, or the dialysis may be run without adding an anticoagulant; however, this increases the risk of a blockage in the system. If patients do not tolerate a heparin-based anticoagulant then alternative anticoagulants can be considered (e.g argatroban or danaparoid), or regional citrate base anticoagulation. Regional citrate base formulations result in the chelation of calcium resulting in an anticoagulant effect; this is not used commonly on account of difficulties in monitoring the effect, and the risk of hypernatraemia, metabolic alkalosis and changes in calcium ion concentration[3],[6]
.
Haemodialysis is usually performed in outpatient dialysis clinics. Patients attend for three sessions per week, with each session lasting three to four hours. This replaces around 10% of a normal kidney’s expected function[4],[5]
. A HD session will, on average, result in the use of 120–160 litres of water to be used for the dialysate. The water must not be contaminated and is purified in most renal units through reverse osmosis[3],
[4]
.
Nocturnal dialysis is an alternative method, with patients having their dialysis overnight for a longer period, normally six to eight hours. This has been shown to have a reduced risk of hypotension as the volume of blood is removed more slowly. There is also evidence to support it reducing hospitalisation and mortality[5]
.
Home HD was only being used by 1,080 patients in the UK in 2012, although this was an increase of nearly 20% from the previous year. Home HD offers the patient a greater level of freedom, reducing the burden of travel and the impact on social or work activities. Patients wanting to have home HD will require additional training to learn the required skills to carry out the process, to be stable on dialysis, have no issues with vascular access, and be independent or have a carer willing to assist with the management plan. The patient’s home will also need to have the space to hold the machine and supplies associated with HD[2]
.
Around one third of patients receiving HD can suffer from postdialysis fatigue where the individual can feel ‘washed out’. It can also occur during the HD session. This has been attributed to a number of different reasons, including the reduced cardiac output, depression, postdialysis hypotension, hypokalaemia and hypoglycaemia[3]
.
Arteriovenous fistulas
Because HD is performed three times a week, it is important the patient has a site that is easily accessible. This is obtained by creating an arteriovenous fistula (AVF), which may be radiocephalic (radial artery to cephalic vein), brachiocephalic (bracial artery to cephalic vein) or brachiobasilic (bracial artery to basilic vein). This involves some of the patient’s arterial blood going into the venous system, which leads to the vessel walls thickening and enlargement of the lumen[6]
.
An AVF normally takes at least six to eight weeks to form and around half will require an additional surgical procedure to ensure it functions correctly[5]
. Upper forearm fistulas are generally created six months before they are used. Alternative vascular access may be required in the interim if dialysis needs to be started[5]
. Generally, if a patient’s renal function is progressively declining, an AVF should be considered when the patient’s eGFR is around 20ml/min/1.73m2 , before dialysis is required[5]
.
A radiocephalic AVF is generally the site of choice, as it means a brachial fistula can still be used if the fistula fails (if a brachial fistula fails a radial fistula cannot be used). However, radiocephalic AVFs have a lower success rate (60%) than the brachiocephalic ones (90%)
[6]
.
In a small number of patients, it will not be possible to use the patient’s own vasculature to create a fistula, and a synthetic graft is required. A synthetic graft involves connecting a vein and artery via a plastic tube; this technique has a higher risk of thrombosis and infection[5]
.
Although an AVF is not required for PD, patients at a high risk of failing on PD may have a fistula created unless a synthetic graft is required.
Peritoneal dialysis
Peritoneal dialysis (PD) can be carried out through automated PD (APD) or continual ambulatory peritoneal dialysis (CAPD). The process involves introducing dialysate into the peritoneum via a permanent indwelling catheter, and using the patient’s peritoneal membrane as a semi-permeable membrane between the peritoneal blood vessels[5]
.
CAPD involves the patient or their carer administering up to 3l of dialysate (warmed to body temperature) into the peritoneal cavity. This volume is dependent on the patient’s body surface area; a body surface area of 1.73m2 requires around 2.5l[5],[6]
. The process is carried out four to five times per day, broken down into shorter dwelling times and an overnight extended dwelling time[5]
. Dialysate remains in the peritoneal cavity for 4–6 hours, after which it is removed via the catheter and a new bag of dialysate is added to the cavity[6]
. Around 8–10l are exchanged per day through CAPD[5]
.
CAPD dialysate will usually contain glucose, which acts as the osmotic agent to remove excess fluid from the body[6]
. As with HD, there are several different dialysates available with varying compositions of electrolytes, and the osmotic agent strength can vary from weak to strong. The osmotic strength of the dialysate can be altered to determine how much fluid removal will take place; for example, a higher concentration of glucose will provide a greater concentration gradient for fluid to be removed[6]
.
APD works in the same way as CAPD, but the majority of the dialysis occurs at night while the patient is sleeping through use of a programmed machine[5]
. It involves on average six changes of fluid (dialysate) over a ten-hour period, all of which are carried out by the dialysis machine. It is more expensive but many patients find it allows them to carry out their normal activities with less restriction[6]
. However, CAPD can be easier for the patient to learn as no machinery is required[6]
.
PD cannot be used indefinitely as patients will develop complications such as peritonitis. Normally, patients are able to remain on PD for up to eight years, although one fifth of individuals continue for more than ten years. Patients should be made aware that they will likely need to change the mode of dialysis at some point to HD[3]
.
A small number of patients receiving PD can suffer from back pain because of the large volume of fluid in the abdomen leading to poor posture. To alleviate this pain, the dialysis regimen can be altered to use less dialysate through the day as long as dialysis adequacy is not affected.
Pain can also occur when fluid is being administered into the abdomen, slowing administration rate should help in most cases but a few will still be affected. The patient may also be in pain when fluids are removed from the abdomen, this may occur in the first weeks of therapy or suggest an infection could be present[3]
. Patients can also have a lowering of their appetite (due to the glucose in the dialysate) and have a sense of fullness in the abdomen due to the large fluid volumes used, which can lead to a state of malnutrition[3]
.
Planning
Clinicians should agree a plan with the patient commencing RRT, which is normally established when the patient is diagnosed as CKD stage 4 (eGFR of 15–30ml/min/1.73m2). However, this may not be possible in patients who present with advanced CKD and require dialysis soon after diagnosis[4]
. Improved diagnosis has reduced the number of patients who present with advanced CKD and require dialysis within 90 days from 23.9% in 2006 to 19.3% in 2012[2]
.
It is important that the nephrology team involves the patient in the decision-making process, equipped with all the information on the advantages and disadvantages of each option, along with advice on which may be the most suitable[5]
.
In practice, some patients find it hard to accept their diagnosis and initially refuse dialysis treatment until their symptoms get progressively worse and they understand dialysis is required[5]
. The patient can go through a multitude of feelings such as grief, denial, anger, bargaining, depression and finally acceptance of condition.
This is a common chain of events, and healthcare professionals should be aware of these stages and aim to reduce the anxiety and fear associated with starting RRT. It is also important to correct any misconceptions the patient has regarding dialysis therapy; for example, many patients believe that dialysis is a highly painful process when in fact pain is not usually experienced[5]
.
Patients at this stage should also have an AVF created to ensure access if dialysis is required.
Practical factors are also important to consider. These include the patient’s social background (e.g. family support, need for carers), occupation (e.g. how many hours, type), and ease and cost of transport to the local hospital[5]
.
Comparison of haemodialysis and peritoneal dialysis
| Haemodialysis | Peritoneal dialysis | |
|---|---|---|
Contraindications | No vascular access
| Abdominal surgery or conditions affecting the abdomen (e.g. diverticular disease) |
Cautions | Patient with severe bleeding disorders | Patients with breathing difficulties |
Past medical history | More suitable for difficult to control type 1 diabetes, although patients with diabetes will have poorer vascular access
| PD (or daily HD) more suitable for patients with cirrhosis or severe cardiomyopathy |
Social | Not recommended if homeless (equipment storage and increased infection risk) | |
Residual renal function | Preserved longer | |
Diet and fluid | Restricted | Less restricted |
Timings | Three times a week (four-hour sessions in hospital) | Greater flexibility, able to dialyse by themselves |
Anticoagulation | Required to keep line patent; heparin can increase risk of retinopathy in diabetes | Not required |
Psychological | Patients can feel more fatigued following a session | Body image may prevent use |
Removal of waste | More effective in removing small molecules than CAPD | Greater clearance of larger solutes but lower clearance of urea |
Cost/year | Higher cost — £35,000 per patient per year for hospital HD | Lower cost — £17,500 per patient per year |
All patients, if appropriate, should be given the opportunity to consider haemodialysis or peritoneal dialysis as their preferred choice of dialysis. In particular, the following patient groups may benefit from PD[7]
:
- Children aged two years or younger;
- Patients with residual renal function;
- Adults without significant pre-existing conditions.
Data suggest there is a lower mortality risk associated with the first two years of PD therapy compared with patients receiving HD. Overall, the mortality rates in HD and PD are similar and dependent on individual factors relating to the patient’s age, past medical history and reason for kidney disease.
Patients with diabetes without significant co-morbidities and younger patients (18–44 years old) have a higher mortality rate when using HD. Risk factors for mortality associated with PD include increasing age, cardiovascular disease, diabetes, poor nutrition and low albumin levels.
- This article was corrected on 24 July 2015 at 14:16 BST. In the article summary, we incorrectly stated that dialysis is usually started in patients with chronic kidney disease (CKD) stage 4 and not stage 5 as it now reads. Similarly, in the box ‘When is dialysis considered?’, it incorrectly stated that dialysis is usually started in patients with chronic kidney disease (CKD) stage 4. A further change was made to the section on peritoneal dialysis has been made to further clarify the position of the cited literature: “Dialysate remains in the peritoneal cavity for around four hours, after which it is removed via the catheter and a new bag of dialysate is added to the cavity [6]” has been changed to “Dialysate remains in the peritoneal cavity for around 4–6 hours, after which it is removed via the catheter and a new bag of dialysate is added to the cavity [6].”
Alan Green is an academic pharmacist practitioner at the University of Sunderland and Gateshead NHS Foundation Trust.
References
[1] Walker R & Whittlesea C. Clinical Pharmacy and Therapeutics, 5th Edition. London: Churchill Livingstone 2011.
[2] The Renal Association. UK Renal Registry 16th Annual Report (2013).
[3] Floege J, Johnson RJ & Feehally J. Comprehensive Clinical Nephrology, 4th edition. London: Elsevier Saunders 2010.
[4] Field M, Pollock C & Harris D. The Renal System: Basic Science and Clinical Conditions, 2nd Edition. London: Churchill Livingstone 2011.
[5] Gilbert SJ & Weiner DE. National Kidney Foundation’s Primer on Kidney Diseases, 6th Edition. London: Elsevier Saunders 2014.
[6] Ashley C & Morlidge C. Introduction to Renal Therapeutics. London: Pharmaceutical Press 2008.
[7] National Institute for Health and Care Excellence. Clinical Guideline (CG125). Peritoneal dialysis: Peritoneal dialysis in the treatment of stage 5 chronic kidney disease. London: NICE 2011.
You might also be interested in…
Dialysis: management
Managing ANCA-associated vasculitis