Shutterstock.com
After reading this article, you should be able to:
- Discuss the causes of electrolyte disturbances and their potential impact on patients;
- Evaluate treatment options for electrolyte disturbances;
- Comply with (or implement) recommended actions from national patient safety alerts relating to electrolyte disturbances.
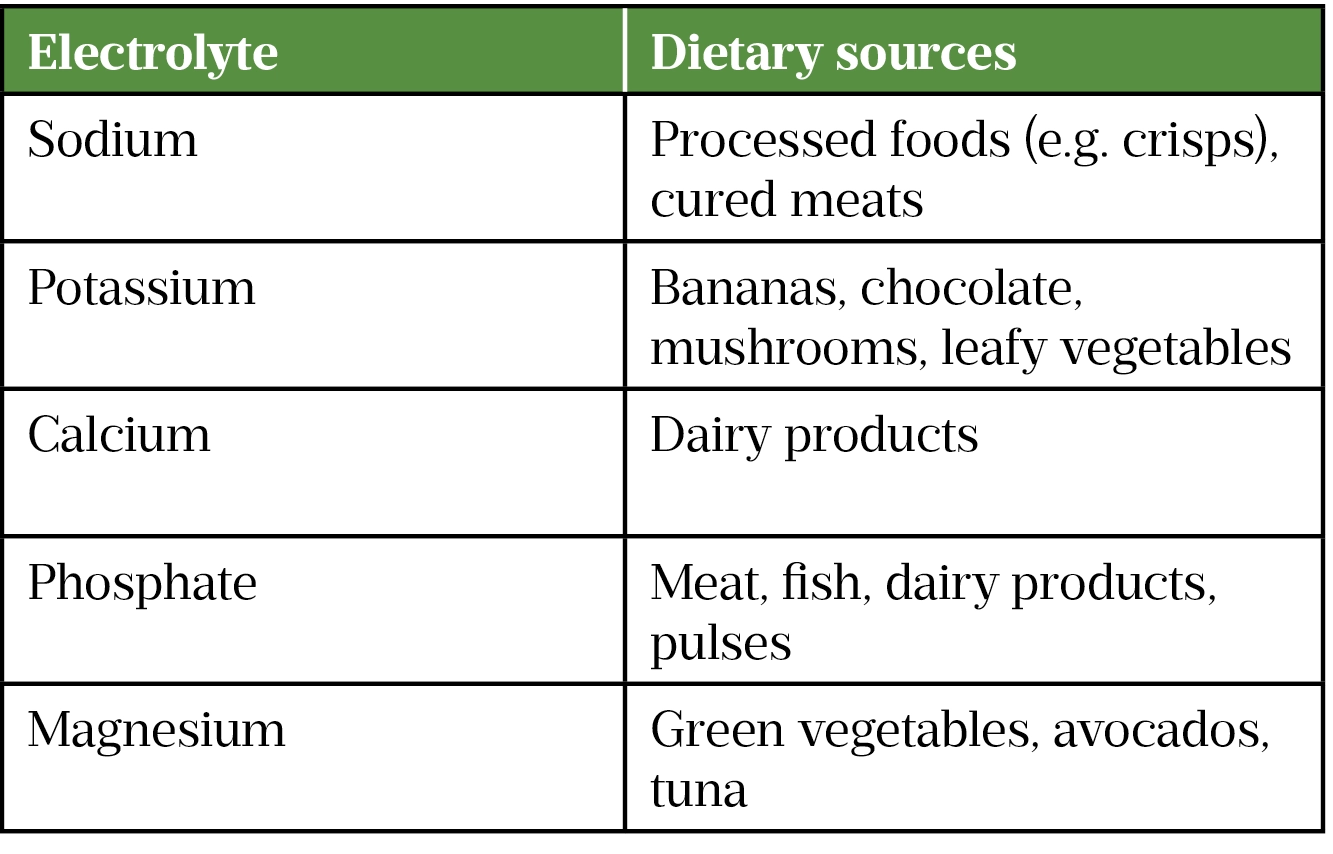
The most common electrolytes in the human body (in tissues and fluids such as blood, urine and sweat) are sodium, potassium, calcium, phosphate and magnesium[1]. Electrolytes play vital roles in nerve conduction, muscle contraction, hormone secretion and enzyme activity[1]. Some bodily functions rely on several electrolytes being within a specified range (e.g. muscle contraction is affected by sodium, potassium, calcium and magnesium concentrations). Food is the main source of electrolytes (see Table 1); however, some medicines have high sodium content (e.g. effervescent tablets, benzylpenicillin and piperacillin/tazobactam)[1,2].
Reference ranges for electrolytes vary between laboratories, as they may use different equipment and testing methods. Regional variations in diet could also influence the perceived normal ranges quoted. Electrolyte disturbances can be caused by infections, medicines, trauma and surgery; poor nutritional intake and malabsorption can also contribute to deficiencies[3]. The exact incidence of electrolyte disturbances in the UK is difficult to define, as individual studies have tended to focus on the occurrence in discrete populations[4,5].
Patients with suspected electrolyte disturbances may require further investigations to confirm the abnormality, evaluate its severity and identify previously undiagnosed disease states that may have contributed to the problem. Managing each disturbance requires identifying and addressing (if possible) the underlying cause(s), along with either supplementation to raise electrolyte levels or specific treatments to lower electrolyte levels, as appropriate. There is no national guidance on the management of all electrolyte disturbances, so local guidelines for each disorder must be consulted.
Patients with mild disturbances may be asymptomatic, but severe disturbances can result in medical emergencies. The detection and management of sodium and potassium imbalances has been the subject of previously issued national patient safety alerts, which highlights the importance of prompt identification and careful management[6–8].
This article discusses the clinical presentation, causes and treatment of sodium, potassium, calcium, phosphate and magnesium disturbances.
Sodium
The major extracellular cation involved in maintaining osmotic pressure and extracellular volume, sodium also plays an important role in neuronal excitability and impulse transmission. The usual reference range is 135–145mmol/L, with serum osmolality being 282–295mOsm/kg of water[9].
Hyponatraemia
Defined as a sodium concentration of <135mmol/L[9], hyponatraemia is the most common electrolyte imbalance, affecting up to 30% of all patients in hospital[10]. It can be classed as either acute (existing <48 hours) or chronic (existing for ≥48 hours). Mild, chronic cases are often asymptomatic, but severe signs and symptoms (e.g. confusion, seizures, cardiorespiratory distress and coma) are more common with rapid-onset cases or when concentrations fall below 125mmol/L. Less serious symptoms seen with acute cases are non-specific and include headache, nausea and vomiting[9].
Hyponatraemia is rarely caused by insufficient dietary sodium; sodium loss and inappropriate secretion of an anti-diuretic hormone (or presence of a compound that mimics its action) are more common contributors[9]. The complexity associated with sodium homeostasis means that cases require careful assessment by considering the patient’s:
- Medical and medication histories;
- Serum sodium concentration and osmolality;
- Urinary sodium concentration and osmolality;
- Glucose and cortisol concentrations;
- Fluid status via blood pressure, pulse, inspection of mucosal membranes, jugular venous pressure and signs of peripheral oedema, ascites and pulmonary congestion[10].
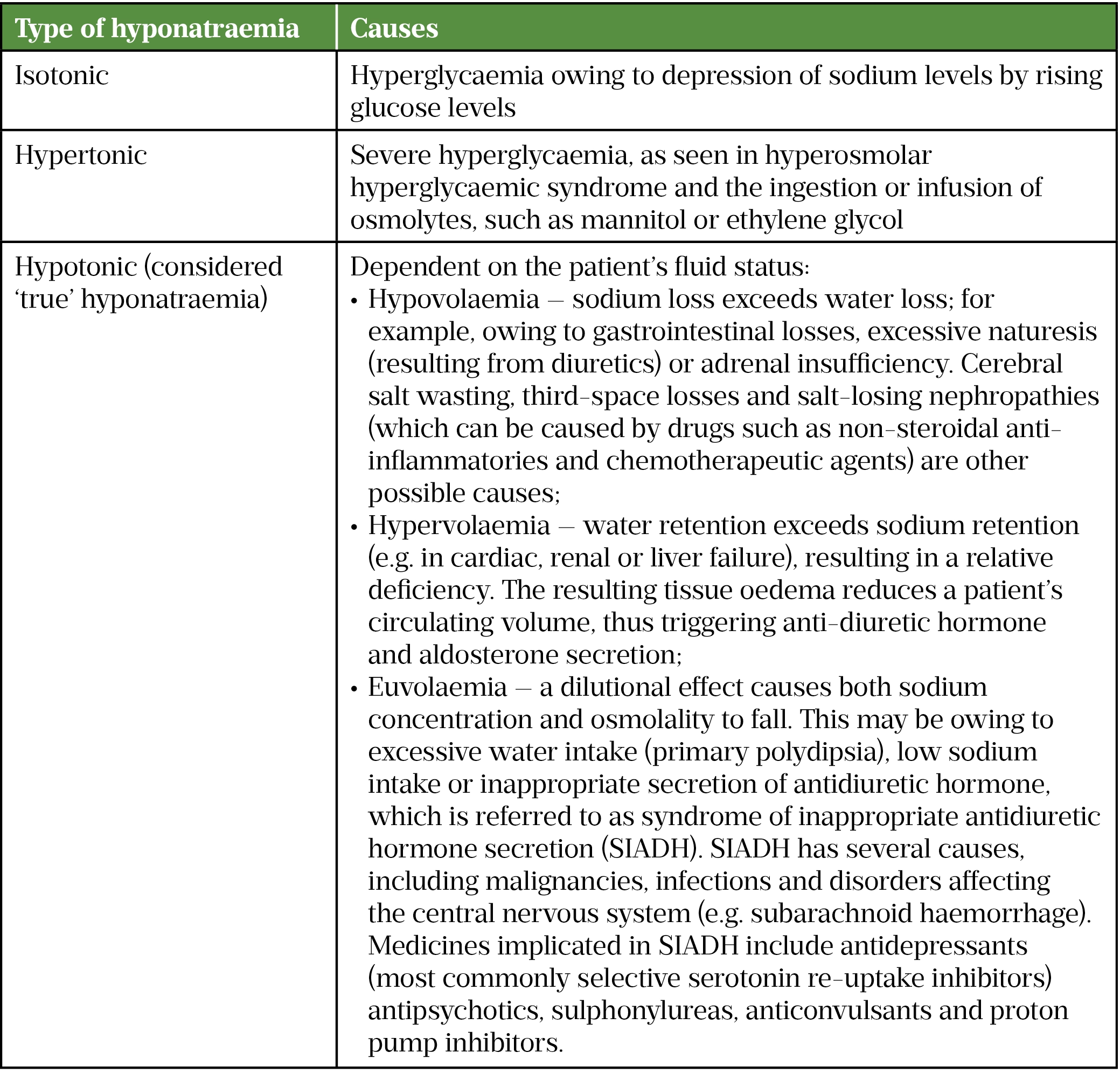
It is beyond the scope of this article to discuss the different subtypes of hyponatraemia, but a summary is provided in Table 2[9–15].
Treatment depends on both the cause and severity of hyponatraemia. However, all patients with serious or life-threatening symptoms require the same initial treatment. The Society of Endocrinology and the European Society of Endocrinology recommend prompt intravenous (IV) treatment with 150mL of 3% (hypertonic) sodium chloride over 20 minutes. Sodium chloride 3% is not commercially available in the UK and therefore hospitals may opt to use a different strength, such as 1.8%. The patient’s serum sodium concentration should be re-checked and another dose given while waiting for the result[11]. These steps can then be repeated twice or until there is a 5mmol/L increase in the serum sodium concentration. Sodium chloride 0.9% can then be used to keep the IV line patent. Local policies should be followed and any decision to initiate hypertonic sodium chloride should be made by a senior clinician with appropriate training and experience.
The increase in serum sodium should be limited to 10mmol/L in the first 24 hours, with an additional increase of 8mmol/L for every subsequent 24-hour period until serum sodium reaches 130mmol/L[10,16]. Further infusions of hypertonic saline can be given if symptoms do not improve, but this must be stopped once there is symptomatic improvement, an increase in serum sodium of 10mmol/L, or if serum sodium reaches 130mmol/L[10,16]. The aim of treatment is to increase serum sodium within the first hour to reduce the risk of cerebral oedema, while simultaneously avoiding over-rapid correction and subsequent demyelination[7,16]. The risks of correcting sodium levels too quickly (resulting in central pontine myelinolysis) were the subject of a national patient safety alert in 2012[7].
Pharmacists need to carefully consider the patient’s diagnosis and the likelihood that the hyponatraemia is drug-induced, alongside the risks and benefits of treatment withdrawal before recommending this as a course of action. Drugs may be incorrectly attributed to hyponatraemia before the subtype (as summarised in Table 2) has been determined. The management of hyponatraemia can be complicated when there is more than one possible cause; therefore, specialist guidance and input need to be sought. For example, the British Society of Gastroenterology’s guidance on managing ascites in patients with cirrhosis discusses when diuretics should be withdrawn and fluid restriction considered when these patients develop hyponatraemia[17]. These patients are most likely to be encountered in hospital where they may have hyponatraemia before or after initiation of spironolactone; a lower serum sodium may be permitted for these patients before diuretics are reduced or withdrawn.
If syndrome of inappropriate antidiuretic hormone secretion is identified as the cause of hyponatraemia, the first-line intervention is fluid restriction[10], with drug treatments reserved for specialist use:
- Demeclocycline is a tetracycline antibiotic known to induce a temporary nephrogenic diabetes insipidus, thus reducing the responsiveness of the kidneys to anti-diuretic hormone (ADH);
- Tolvaptan is a selective vasopressin receptor 2 antagonist that blocks the binding of ADH to this receptor in the kidneys, preventing water reabsorption[12,18].
Hypernatraemia
Hypernatraemia is a common presentation in older hospitalised patients (owing to inadequate water intake relative to water losses, such as diarrhoea, vomiting or fever), as well as those with altered mental status (caused by either lack of access to water or impaired thirst response)[16]. Drug-related causes include corticosteroids, fosfomycin, lithium, contraceptives and products with a high sodium content[9]. In most patients, sustained hypernatraemia does not occur as fluid loss stimulates thirst, and the subsequent intake of fluids corrects the serum sodium and osmolality[13]. The excessive fluid losses observed in some conditions (e.g. hyperglycaemic hyperosmolar syndrome) and hypertonic sodium administration can also lead to hypernatraemia. Pharmacists also need to be aware of the impact of heat-related illnesses on fluid balance and sodium levels, and signpost patients to NHS resources when a heatwave is anticipated.
Management involves treating the underlying cause and correcting the hypertonicity. Correcting hypertonicity is achieved with appropriate fluid replacement, preferably using oral or enteral fluids[12]. Suitable IV fluids include dextrose 5% and hypotonic saline (e.g. 0.18%). If the hypernatraemia has developed over a short period (i.e. a few hours), rapid correction can be used without risking cerebral oedema; an appropriate rate of decrease in serum sodium is 1mmol/L/hr[19]. If hypernatremia is present for more than a few hours, or the duration is unknown, a slower rate of correction is necessary to avoid cerebral oedema and central pontine myelinolysis[19,20]. In those circumstances, the rate of serum sodium correction should not exceed 0.5mmol/L/hr, with a recommended target fall of 10mmol/L/day until serum sodium reaches the top end of the target range[20].
Potassium
The major intracellular cation, potassium’s main role is to maintain resting membrane potentials (e.g. in the heart). The reference range is usually 3.5–5mmol/L[9].
Hypokalaemia
Usually defined as a serum potassium level less than 3.5mmol/L, hypokalaemia can be caused by vomiting, diarrhoea, Cushing’s syndrome, magnesium deficiencies and medicines that promote either its excretion (e.g. loop and thiazide diuretics, laxatives) or movement into cells (e.g. beta-2 agonists and insulin)[9]. Patients receiving insulin should be advised on how to ensure an adequate dietary intake of potassium intake — referral to a dietician may be needed if patients develop recurrent hypokalaemia. Symptoms include muscle weakness, paralytic ileus and cardiac arrhythmias. Hypokalaemia can also predispose patients to digoxin toxicity owing to reduced competition at the sodium–potassium pump. A level below 2.5mmol/L requires urgent treatment[21].
If the oral route is available and there are no concerns about absorption, then oral replacement therapy (usually either two Sando-K tablets [potassium chloride, potassium bicarbonate; Alturix] two to three times per day or Kay-Cee-L liquid 10mL [potassium chloride; Geistlich Sons] three times per day, both for three days) is usually sufficient for mild cases (i.e. levels above 2.5mmol/L and asymptomatic)[22]. Dispersible potassium tablets have poor palatability but can be mixed with squash or juice to overcome this[23]. Modified-release potassium tablets are rarely used nowadays, as there is a risk of serious gastrointestinal irritation if they become lodged in abnormalities of the gastrointestinal tract[24]. IV potassium is needed if the oral route is unavailable, if there are concerns about absorption, or if a patient’s potassium level is below 2.5mmol/L[25]. IV potassium is considered a high-risk drug owing to the risks of:
- Cardiac arrhythmia if it is administered too quickly — therefore the maximum rate of potassium infusion is 20mmol/hour. However, on non-critical-care wards, the maximum rate of infusion is often 10mmol/hour;
- Irritation (and even extravasation) if a solution that is too concentrated is administered via peripheral veins. Therefore, the usual maximum concentration of potassium for peripheral administration is 40mmol/500mL (although limiting this to 40mmol/L is preferred); potassium chloride solutions containing 40mmol/100mL must be administered via a central line and tend to be restricted to critical care areas[25,26].
Following several reports of errors involving potassium chloride (including inadvertent IV bolus administration) and the subsequent publishing of a patient safety alert by the National Patient Safety Agency, many trusts stopped storing ampoules of potassium chloride on wards and replaced them with ready-made bags of IV potassium solutions[25]. However, these need to be stored separately from other IV fluids to avoid incorrect selection. Mis-selection of a strong potassium solution is one of the five medicine-related ‘Never events’ on the list published by NHS Improvement[27].
Hyperkalemia
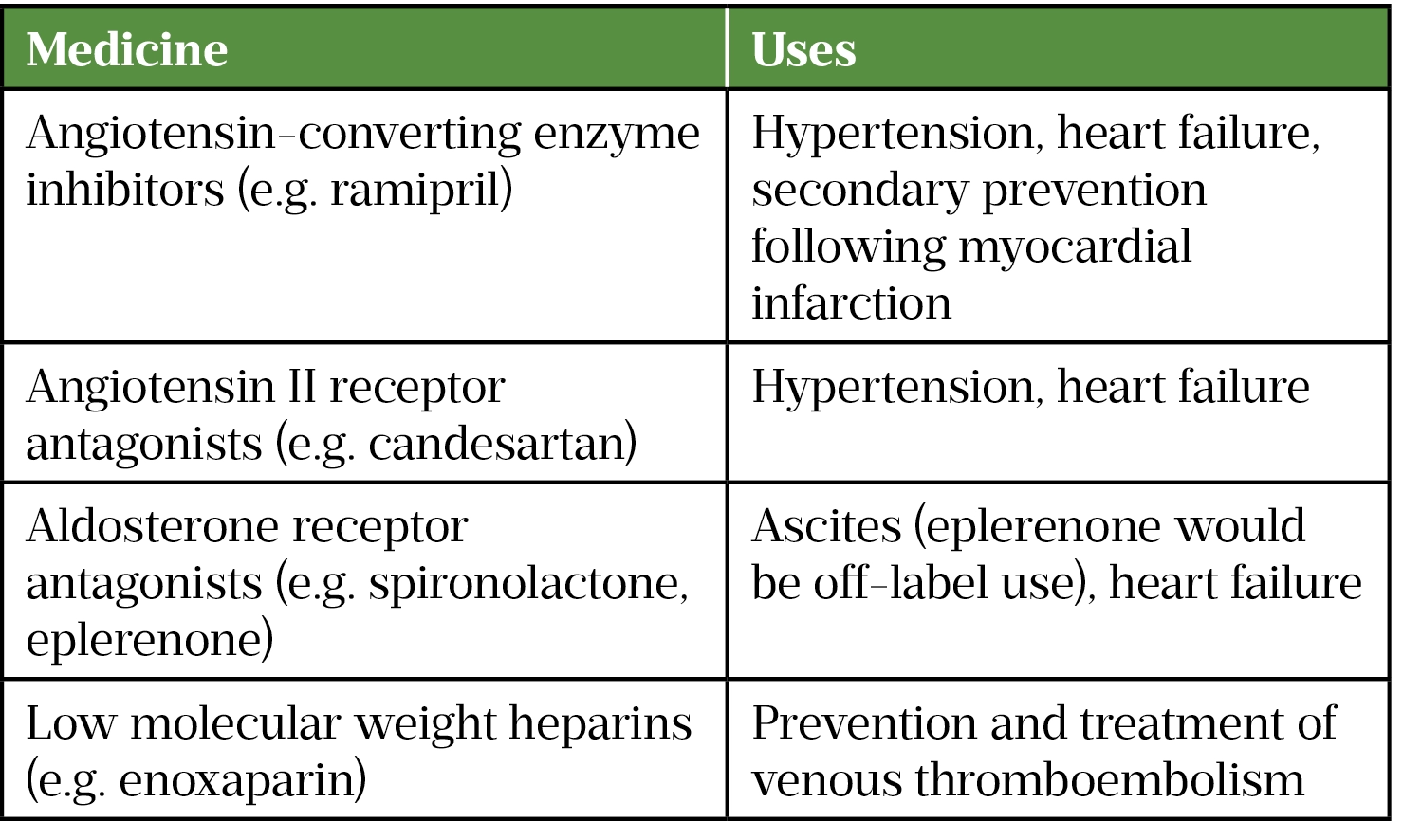
Hyperkalemia can be caused by renal impairment (both acute and chronic), tumour lysis syndrome and medicines that cause retention of potassium (see Table 3)[28].
Treatment depends on the potassium level reported and whether the patient has any concerning signs or symptoms (e.g. electrocardiogram [ECG] changes, chest pain, fast, irregular pulse). A potassium level above 6.5mmol/L is considered a medical emergency owing to the risk of ventricular tachycardia and death[21]. In these cases, or where there are concerning signs or symptoms, patients require an IV bolus of 10mL of calcium gluconate 10% to stabilise the myocardium[29]. Drugs that can raise potassium levels should be stopped where possible and local policies should be consulted for treatments that will lower potassium levels. For example:
- Salbutamol nebules and infusions of insulin in glucose promote the movement of potassium into cells. These treatments act quickly but there is a risk of rebound hyperkalaemia once they are stopped, as potassium may leak back out of cells;
- Agents that help with potassium excretion:
- Calcium resonium (15g four times per day) acts as an ion exchange resin and is usually used for milder cases (serum potassium <6mmol/L). This treatment has poor palatability and can cause constipation. A stool softener, such as lactulose, is given to prevent this (macrogols are best avoided since they contain potassium). Treatment can be stopped when a patient’s potassium reaches 5mmol/L;
- The National Institute for Health and Care Excellence (NICE) has recently approved two new treatments for hyperkalaemia — sodium zirconium and patiromer.
- In severe cases, patients may require haemodialysis to remove the excess potassium[28,30–33].
Patients being managed for severe hyperkalaemia require serum potassium checking 1 hour, 2 hours , 4 hours, 6 hours and 24 hours after treatment is started[34].
Sodium zirconium and patiromer have been approved by NICE for the management of acute and chronic hyperkalaemia, and for use in patients with heart failure who cannot tolerate target doses of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II-receptor blockers (ARBs) because of the development of hyperkalaemia[32,33]. Patients with heart failure could benefit significantly from these treatments, since studies have shown better outcomes are achieved if patients are titrated to target doses of ACEIs or ARBs[35].
Calcium
Found mostly in bone (99% of total), calcium also plays a role in muscle contraction. The usual reference range is 2.12–2.6mmol/L, with levels adjusted for albumin level. It is regulated by the parathyroid hormone (PTH) via a negative feedback loop[9].
Hypocalcaemia
Hypocalcaemia most commonly results from either hypoparathyroidism (often following damage or removal of the parathyroid gland following neck surgery) or a deficiency of, or inability to activate, vitamin D[13]. Magnesium deficiency, pancreatitis and rhabdomyolysis may also be implicated and drug-induced causes include aminoglycosides, bisphosphonates, cisplatin, loop diuretics and phenytoin[19]. Symptoms of hypocalcaemia are not usually seen until levels fall below 1.9mmol/L, and include tingling and numbness of fingers, toes, and lips, cramps, tetany and seizures. Accurate diagnosis of the underlying cause requires measuring serum calcium, magnesium, PTH and vitamin D levels[36]. The management of the different types of hypoparathyroidism is beyond the scope of this article.
Treatment is centred around supplementation with calcium carbonate (although citrate can be used) and vitamin D, with doses based on the severity and whether the presentation is acute or chronic. The amount of calcium supplementation required varies enormously, with some patients requiring 9g per day[37]. Asymptomatic acute hypocalcaemia (with serum calcium levels >1.9mmol/L) can be managed with oral calcium supplementation (e.g. two Calvive 1000 effervescent tablets [calcium carbonate, calcium lactate gluconate; GSK] twice daily)[36]. Oral supplementation often tastes unpleasant, so pharmacists can help optimise adherence to calcium therapy by choosing formulations that are acceptable to the patient.
Acute, severe hypocalcaemia (serum calcium <1.9mmol/L or any level of deficiency with symptoms) is a medical emergency and requires treatment with IV calcium. In addition to treating the underlying cause, the Society of Endocrinology recommends an initial dose of 10–20mL calcium gluconate 10% in 50–100mL dextrose 5% over 10 minutes with ECG monitoring, which can be repeated until the patient is asymptomatic[36]. This should be followed by an infusion of 100mL calcium gluconate 10% in 1L sodium chloride 0.9% or glucose 5% infused at 50–100mL/hr, adjusted to achieve normocalcaemia[36]. However, local policies for calcium infusions may differ from these recommendations and should be followed as appropriate.
As side effects are associated with excessive calcium intake, many practitioners will try to limit the amount of calcium supplementation by being more proactive with the use of active vitamin D[38]. Active vitamin D is used as PTH is involved in the activation process. Calcitriol 0.25–2 micrograms once or twice daily and alfacalcidol 0.5–4 micrograms once daily are recommended by the European Society of Endocrinology because of their fast onsets of action (one to two days)[39]. Ergocalciferol and colecalciferol 25,000–200,000 units daily are also options, although they have a slow onset of action (10–14 days) and should generally be used only when active vitamin D metabolites are not available[38]. Serum calcium should be monitored as often as weekly during dose adjustments, but can be reduced to twice yearly in patients on stable regimes[38]. In patients with hypercalciuria, (i.e. excess urinary calcium), a thiazide diuretic can be prescribed to promote renal tubular calcium retention, but monitoring of other electrolytes and fluid status is needed[37,38].
Hypercalcaemia
Hypercalcaemia most commonly results from hyperparathyroidism or malignancy, with rarer causes including familial hypocalciuric hypercalcaemia. Drug-induced causes include vitamin D intoxication, thiazide diuretics and lithium[13]. Symptoms of hypercalcaemia are uncommon until levels rise above 3mmol/L, but include polyuria, polydipsia, vomiting, constipation, abdominal pain and confusion. Treatment depends on the underlying cause; offending drugs should be withdrawn, if possible. A parathyroidectomy is the only definitive treatment for primary hyperparathyroidism.
Acute hypercalcaemia with a serum calcium level of >3mmol/L requires prompt treatment, since a level of >3.5mmol/L can cause dysrhythmias and coma [26]. The mainstay of treatment is rehydration: 4–6L of sodium chloride 0.9% is usually needed in 24 hours; loop diuretics can be given if fluid overload develops[26]. If further treatment is required after fluids, IV bisphosphonates are given (e.g. zoledronic acid 4mg over 15 minutes, pamidronate 30–90mg at 20mg/hr and ibandronic acid 2–4mg), with doses adjusted on the basis of renal function[40]. Serum calcium should be monitored, as maximal response can take several days and hypocalcaemia can occur, especially if the patient is vitamin D deficient or has supressed PTH[13]. Glucocorticoids (e.g. prednisolone 40mg once daily) are another option in patients with lymphoma, chronic granulomatous disease or vitamin D toxicity, since they inhibit 1,25(OH)D (the active form of vitamin D) production and are usually effective within two to four days[40]. Specialists may also use cinacalcet, denosumab and calcitonin.
Phosphate
The majority of the body’s phosphate (85%) is found in bone, with most of the remaining portion being intracellular (and involved in energy production). The usual reference range is 0.8–1.44mmol/L[9].
Hypophosphataemia
Hypophosphataemia is often asymptomatic in chronic or mild cases (0.6–0.8mmol/L)[9]. At levels below this range, symptoms such as muscle weakness, bone pain and confusion may be seen. Levels below 0.3mmol/L can cause disorientation, seizures and congestive heart failure. The causes of hypophosphataemia can be divided into three categories:
- Poor intake (e.g. malnutrition, reduced absorption owing to diarrhoea or excessive phosphate binder use, vitamin D deficiency);
- Increased excretion owing to hyperparathyroidism or drugs that cause renal phosphate leak (e.g. aminoglycosides, cisplatin). A 2020 drug safety alert highlighted the risk of hypophosphataemia in patients receiving high-dose or long-term treatment with ferric carboxymaltose;
- Shifts to the intracellular compartment (e.g. diabetic ketoacidosis [because of effects of supplementary insulin], leukaemia and refeeding syndrome). It is important to address hypophosphataemia in malnourished patients before feeding is commenced, since phosphate is needed in glucose metabolism[1,4,41,42].
Oral Phosphate Sandoz supplements (sodium acid phosphate; Atriux) are often sufficient for moderate deficiencies (0.3–0.59mmol/L), but general management of hypophosphataemia (i.e. not because of vitamin-D-resistant rickets) is an off-label use of this product[43,44]. Severe deficiencies (or where the oral route is not available) require IV replacement with ready-made infusion bags (e.g. Addiphos 20mmol [potassium dihydrogen phosphate, disodium phosphate dihydrate, potassium hydroxide; Fresenius Kabi] in 500mL glucose 5% or phosphate polyfusor — doses vary between trusts and may be repeated if follow up levels still indicate a deficiency. Since Addiphos and phosphate polyfusors contain potassium, sodium glycerophosphate infusions may be used if potassium needs to be restricted[43].
Hyperphosphataemia
Usually seen in the more advanced stages of chronic kidney disease, hyperphosphataemia can also develop acutely if renal function is sufficiently diminished. Symptoms include nausea and pruritus and, if raised levels are sustained, secondary hyperparathyroidism.
Treatment involves avoiding or limiting food rich in phosphate (e.g. dairy products, meat, fish, pulses) with a specialist dietician providing support to patients. Phosphate binders (e.g. calcium acetate, sevelamer and lanthanum) taken with meals help reduce phosphate absorption and doses are adjusted on the basis of subsequent levels[45].
Magnesium
Mainly found in bones, muscles and soft tissue, magnesium acts as an important co-factor for various enzymes and adenosine triphosphate. It therefore plays an important role in nerve conduction, muscle contraction and metabolic processes. The usual reference range is 0.7–1mmol/L[9].
Hypomagnesaemia
Hypomagnesaemia is commonly encountered in critically ill patients (owing to infection, surgery or excessive gastrointestinal losses) and those with malnutrition or absorption problems. The drugs implicated most commonly are calcineurin inhibitors (e.g. ciclosporin) and amphotericin B, but laxatives and aminolgycosides can also affect magnesium levels[9].
Patients with levels in the 0.4–0.7mmol/L range may not require treatment unless symptomatic, but patients with levels <0.4mmol/L should be prescribed replacement therapy. Oral replacement therapies are usually first line and include one to two Magnaspartate 243mg sachets (10mmol) (magnesium aspartate dihydrate; Kora Healthcare) daily in divided doses and magnesium glycerophosphate tablets (24mmol) daily in divided doses (i.e. two tablets three times per day)[46]. However, oral replacement can cause gastrointestinal problems (and contribute to further losses) and absorption may be compromised in patients with short bowel syndrome. For IV replacement, the British National Formulary recommends up to 40g (160mmol) given over a period of up to 5 days[47]. The licensed dose for single infusions is 20mmol of magnesium sulphate in 1L of sodium chloride 0.9% given over three hours, but some centres may use longer infusion times (which may also be more suitable for non-emergency cases)[46].
Hypermagnesaemia
Compared with other electrolyte disturbances, hypermagnesaemia is rarely encountered and is usually caused by excess ingestion of magnesium. Severe toxicity requires 10–20mL of IV calcium gluconate 10% alongside circulatory and respiratory support. Furosemide can help increase magnesium excretion if the patient’s renal function is adequate, but severe cases may require haemodialysis[48].
Summary and best practice
This article has highlighted the significance of electrolyte disturbances and how national patient safety alerts have been issued to help improve detection and ensure they are managed safely. Therefore, keeping up to date with changes in practice, by subscribing to relevant alerts and bulletins, such as the Medicines and Healthcare products Regulatory Agency’s Drug Safety Update, is vital.
Pharmacists in patient-facing roles need to be vigilant about the potential for prescribed medicines to cause electrolyte disturbances and take steps to ensure appropriate monitoring plans are in place. As there is a lack of national guidance on the management of all electrolyte disturbances, prescriptions for treatments of these disorders should be checked against local guidelines. Drug-induced electrolyte disturbances that result in hospitalisation should be reported via the Medicines and Healthcare products Regulatory Agency’s Yellow Card Scheme. Pharmacists working in hospitals will encounter patients with electrolyte disturbances on a regular basis, whereas those working in general practice will see cases less often. However, they may have responsibility for requesting and reviewing electrolyte results for patients when conducting structured medication reviews. Community pharmacists can also identify patients requiring electrolyte monitoring (e.g. those prescribed a combination of a diuretic and digoxin) by asking patients who are collecting repeat prescriptions when they last had blood tests. Some over-the-counter medicines have a high sodium content (e.g. effervescent tablets) and excessive use (or abuse) of laxatives can result in various electrolyte deficiencies (especially potassium, phosphate and magnesium); therefore, obtaining relevant information before providing such products is vital.
- 1Fluid and Electrolyte Balance. MedlinePlus. 2016.https://medlineplus.gov/fluidandelectrolytebalance.html (accessed Aug 2022).
- 2Marsden A. Considering sodium content of medicines. Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/considering-sodium-content-of-medicines/ (accessed Aug 2022).
- 3Holland K. All About Electrolyte Disorders. Healthline. 2019.https://www.healthline.com/health/electrolyte-disorders (accessed Aug 2022).
- 4Liamis G, Rodenburg EM, Hofman A, et al. Electrolyte Disorders in Community Subjects: Prevalence and Risk Factors. The American Journal of Medicine. 2013;126:256–63. doi:10.1016/j.amjmed.2012.06.037
- 5Tazmini K, Nymo SH, Louch WE, et al. Electrolyte imbalances in an unselected population in an emergency department: A retrospective cohort study. PLoS ONE. 2019;14:e0215673. doi:10.1371/journal.pone.0215673
- 6Patient Safety Alert: Potassium Solutions. Institute for Healthcare Improvement. 2002.https://www.ihi.org/resources/Pages/Tools/PatientSafetyAlertPotassiumSolutions.aspx (accessed Aug 2022).
- 7Signal Alert: Risk of Harm from CPM Syndrome Following Rapid Correction of Sodium. National Patient Safety Agency. 2012.www.google.co.uk (accessed Aug 2022).
- 8Patient Safety Alert: Resources to support safe and timely management of hyperkalaemia. NHS England. 2019.https://www.england.nhs.uk/publication/patient-safety-alert-safe-and-timely-management-of-hyperkalaemia/ (accessed Aug 2022).
- 9Warburton J, Beale L. Laboratory data. In: Whittlesea C, Hodson K, eds. Clinical Pharmacy and Therapeutics. Elsevier 2019. 81–104.
- 10Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. European Journal of Endocrinology. 2014;170:G1–47. doi:10.1530/eje-13-1020
- 11Ball S. How I approach hyponatraemia. Clin Med. 2013;13:291–5. doi:10.7861/clinmedicine.13-3-291
- 12Hyponatraemia. National Institute for Health and Care Excellence. 2020.https://cks.nice.org.uk/topics/hyponatraemia/ (accessed Aug 2022).
- 13Wass J, Owen K, editors. Oxford Handbook of Endocrinology and Diabetes. 3rd ed. Oxford University Press 2014.
- 14Jones C, Jones A, Lloyd F. Investigation and Management of Hyponatraemia in Adults in Primary Care. York and Scarborough Teaching Hospitals NHS Foundation Trust. 2019.https://www.yorkhospitals.nhs.uk/seecmsfile/?id=3852 (accessed Aug 2022).
- 15Falhammar H, Lindh JD, Calissendorff J, et al. Differences in associations of antiepileptic drugs and hospitalization due to hyponatremia: A population–based case–control study. Seizure. 2018;59:28–33. doi:10.1016/j.seizure.2018.04.025
- 16Ball S, Barth J, Levy M, et al. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of severe symptomatic hyponatraemia in adult patients. Endocrine Connections. 2016;5:G4–6. doi:10.1530/ec-16-0058
- 17Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2020;70:9–29. doi:10.1136/gutjnl-2020-321790
- 18Farrington M, Brown M, Sharma P. Antibacterial drugs. In: Bennett P, ed. Clinical Pharmacology. London: : Churchill Livingstone 2012. 173–190.
- 19Maggs FG. The management of patients presenting with hypernatraemia: is aggressive management appropriate? Clin Med. 2014;14:260–3. doi:10.7861/clinmedicine.14-3-260
- 20Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–9. doi:10.1056/nejm200005183422006
- 21Wilkinson I, Raine T, Wiles K, et al. Oxford Handbook of Clinical Medicine. 10th ed. Oxford University Press 2019.
- 22Potassium chloride. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/drugs/potassium-chloride/ (accessed Aug 2022).
- 23Potassium chloride for potassium depletion. Medicines for Children. 2019.https://www.medicinesforchildren.org.uk/medicines/potassium-chloride-for-potassium-depletion/ (accessed Aug 2022).
- 24Sandor F. Complications of ‘slow-K’ therapy. J R Coll Gen Pract 1976;26:595–8.https://www.ncbi.nlm.nih.gov/pubmed/966209
- 25Potassium (intravenous) policy. 7th ed. Wirral University Teaching Hospitals NHS Foundation Trust 2019.
- 26Burgess A. How should intravenous (IV) potassium chloride be administered in adults? Specialist Pharmacy Service. 2020.https://www.sps.nhs.uk/articles/how-should-intravenous-iv-potassium-chloride-be-administered-in-adults/ (accessed Aug 2022).
- 27Never Events list 2018. NHS Improvement. 2021.https://www.england.nhs.uk/wp-content/uploads/2020/11/2018-Never-Events-List-updated-February-2021.pdf (accessed Aug 2022).
- 28McGuire M, Pennathur S. Hyperkalemia. The Saint-Chopra Guide to Inpatient Medicine. 2018;:265–8. doi:10.1093/med/9780190862800.003.0046
- 29Management of Acute Hyperkalaemia in Adults. Wirral University Teaching Hospitals NHS Foundation Trust 2022.
- 30Ahee P. The management of hyperkalaemia in the emergency department. Emergency Medicine Journal. 2000;17:188–91. doi:10.1136/emj.17.3.188
- 31Maxwell A, Linden K, O’Donnell S, et al. Management of hyperkalaemia. J R Coll Physicians Edinb. 2013;43:246–51. doi:10.4997/jrcpe.2013.312
- 32Sodium zirconium cyclosilicate for treating hyperkalaemia. National Institute for Health and Care Excellence. 2022.https://www.nice.org.uk/guidance/ta599/chapter/2-Information-about-sodium-zirconium-cyclosilicate (accessed Aug 2022).
- 33Patiromer for treating hyperkalaemia. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ta623 (accessed Aug 2022).
- 34Alfonso A, Harrison A, Baines R, et al. Treatment of acute hyperkalaemia in adults. UK Kidney Association. 2020.https://ukkidney.org/health-professionals/guidelines/treatment-acute-hyperkalaemia-adults (accessed Aug 2022).
- 35Khan MS, Fonarow GC, Ahmed A, et al. Dose of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers and Outcomes in Heart Failure. Circ: Heart Failure. 2017;10. doi:10.1161/circheartfailure.117.003956
- 36Turner J, Gittoes N, Selby P, et al. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of acute hypocalcaemia in adult patients. Endocrine Connections. 2016;5:G7–8. doi:10.1530/ec-16-0056
- 37Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of Hypoparathyroidism with PTH(1–84): A Prospective Four-Year Investigation of Efficacy and Safety. The Journal of Clinical Endocrinology & Metabolism. 2013;98:137–44. doi:10.1210/jc.2012-2984
- 38Brandi ML, Bilezikian JP, Shoback D, et al. Management of Hypoparathyroidism: Summary Statement and Guidelines. The Journal of Clinical Endocrinology & Metabolism. 2016;101:2273–83. doi:10.1210/jc.2015-3907
- 39Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. European Journal of Endocrinology. 2015;173:G1–20. doi:10.1530/eje-15-0628
- 40Walsh J, Gittoes N, Selby P, et al. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of acute hypercalcaemia in adult patients. Endocrine Connections. 2016;5:G9–11. doi:10.1530/ec-16-0055
- 41Ferric carboxymaltose (Ferinject▼): risk of symptomatic hypophosphataemia leading to osteomalacia and fractures. Medicines and Healthcare products Regulatory Agency. 2020.https://www.gov.uk/drug-safety-update/ferric-carboxymaltose-ferinject-risk-of-symptomatic-hypophosphataemia-leading-to-osteomalacia-and-fractures (accessed Aug 2022).
- 42Nutritional Assessment. British Association for Parenteral and Enteral Nutrition. 2016.https://www.bapen.org.uk/nutrition-support/assessment-and-planning/nutritional-assessment?start=2 (accessed Aug 2022).
- 43Management of hypophosphataemia. Wirral University Teaching Hospitals NHS Foundation Trust 2020.
- 44Phosphate Sandoz. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/958 (accessed Aug 2022).
- 45Chronic kidney disease: assessment and management. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng203 (accessed Aug 2022).
- 46Abbott D. How is acute hypomagnesaemia treated in adults? Specialist Pharmacy Service. 2021.https://www.sps.nhs.uk/articles/how-is-acute-hypomagnesaemia-treated-in-adults/ (accessed Aug 2022).
- 47Magnesium sulfate. National Institute for Health and Care Excellence. 2022.https://bnf.nice.org.uk/drugs/magnesium-sulfate/#indicationsAndDoses (accessed Aug 2022).
- 48Yu A, Gupta A. Hypermagnesemia: Causes, symptoms, and treatment. UpToDate. 2022.https://www.uptodate.com/contents/hypermagnesemia-causes-symptoms-and-treatment#H133515692 (accessed Aug 2022).


