This content was published in 2010. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
There is now a wide range of treatments available for patients with epilepsy. The newer antiepileptic drugs (AEDs) are more acceptable in terms of adverse effects and provide additional options for patients not responding to first-line treatments. Treatment decisions should be made jointly between the patient/carer and a specialist, and monitoring of treatment response, side effects and drug interactions is essential.
Clinicians should discuss the possibility of discontinuing AED treatment in adults who have been seizure-free for at least two years. A decision to discontinue treatment should be based on the patient’s risk of seizures, lifestyle, prognosis and seizure syndrome.
The mainstay of treatment for epilepsy is with antiepileptic drugs (AEDs). The benefits of these medicines in terms of preventing or reducing seizures need to be weighed carefully against their possible adverse effects. The aim of AED treatment is to control seizures as quickly as possible — preferably using monotherapy — with no or minimal adverse effects and without impact on the patient’s quality of life. Effective pharmacological treatment with AEDs requires a reasonable certainty of the diagnosis of epilepsy or seizure type.
The therapeutic response to AEDs varies with seizure type. Indeed, some AEDs can worsen certain types of seizures. On the whole, about 50% of newly diagnosed patients become seizure-free within one year of starting treatment with an AED.1,2
This article will focus on the pharmacological treatments used in managing epilepsy and the associated drug monitoring.
Initiating treatment
The decision to start an AED should be made jointly by the patient or his or her carers and a specialist following an informed discussion of the risks and benefits of treatment. Treatment with an AED is generally initiated after a second epileptic seizure; however, consideration should be given to starting treatment after a single unprovoked seizure if:
- The patient has a neurological deficit
- An electroencephalogram shows unequivocal epileptic activity
- The patient or carers consider the risk of having a further seizure unacceptable
- Brain imaging shows a structural abnormality
Initial treatment should be with a single AED, started at a low dose and increased gradually until seizures are controlled or significant adverse effects occur. The maintenance dose will be determined by the individual’s response to therapy and the balance between control of seizures and adverse effects.3–7
Choosing an AED
In general, AEDs exert their action by: mimicking or increasing the effects of gamma-aminobutyric acid or other inhibitory neurotransmitters; or limiting neuronal firing by acting on voltage-gated sodium or calcium channels.
Antiepileptic medication should be tailored according to individual patient requirements. When choosing an AED, clinicians should consider:
- Seizure types and epilepsy syndrome
- Patient preference
- Age
- Childbearing potential
- Other comorbidities
- Likely interactions with concomitant medicines
- Formulations available
Guidance published by the National Institute for Health and Clinical Excellence in 2004 recommends using older AEDs as first-line monotherapy, with the newer medicines used as adjuncts if seizures continue. The newer drugs are recommended as options for first-line treatment if the older drugs are contraindicated because of adverse effects or interactions with existing medicines and where applicable for women of childbearing age. Once seizures are controlled, drug therapy should be continued until the patient has been seizure-free for at least two years.
Older AEDs
In the UK the most commonly prescribed AEDs are carbamazepine and sodium valproate, although phenytoin is still widely used.
Carbamazepine
Carbamazepine is the drug of choice for treating simple and complex seizures and for tonicclonic seizures secondary to a focal discharge. It is not usually effective in absence and myoclonic seizures. Baseline liver function tests (LFTs), full blood count (including platelet count) and urea and electrolytes should be obtained before starting treatment and repeated six-monthly thereafter. LFTs are particularly important for patients with a history of liver disease and for elderly patients. Carbamazepine should be withdrawn immediately if acute liver disease develops or if liver dysfunction worsens.
It is essential to start at a low dose (100–200mg once or twice a day orally for adults, lower for the elderly) and increase slowly at increments of 100–200mg every two weeks. The dose is titrated upwards until seizures are controlled or the patient is not able to tolerate side effects. Plasma drug monitoring may be used to guide dosing of carbamazepine; however, the maintenance dose should be determined by patient response not by drug levels.
The most common dose-related side effects include fatigue, nausea, blurred vision and ataxia. These are usually lessened by dose-reduction or changing to a modifiedrelease formulation. Rare but serious adverse effects include agranulocytosis, Stevens-Johnson syndrome (SJS), aplastic anaemia, thrombocytopenia and hepatic impairment. In addition to carefully monitoring patients’ clinical state, clinicians should educate patients and carers on early signs indicating haematological, dermatological or hepatic toxicities (eg, fever, sore throat, rash, mouth ulcers, easy bruising or bleeding) and advise them to report these immediately so carbamazepine can be withdrawn promptly.
The allele HLA-B1502 in patients of Han Chinese and Thai origin has been shown to predict strongly the risk of developing carbamazepine-associated SJS. Where possible, patients of these ethnic groups should be screened for this allele before initiating treatment with carbamazepine. If tested positive, carbamazepine should be avoided unless there is no other therapeutic option. It should be noted that patients who test positive for HLA-B1502 may be at increased risk of hypersensitivity from other AEDs. Cross-sensitivity occurs between the AEDs in up to 70% of patients. It is probably advisable to avoid other AEDs also known to cause SJS, namely lamotrigine, phenytoin and phenobarbital.
Being an enzyme inducer, carbamazepine has a high potential for interactions; possible drug-drug interactions should be monitored closely and patients counselled appropriately.
“Older” antiepileptic drugs and effects on bone
Phenytoin, primidone, carbamazepine and sodium valproate have been linked with reduced bone mineral density among patients, leading to an increased risk of osteopenia, osteoporosis and fractures resulting from falls. Healthcare professionals have been advised in the April 2009 Drug Safety Update (published by the Medicines and Healthcare products Regulatory Agency) to consider prescribing supplementary vitamin D — especially for at-risk individuals who receive long-term treatment.8
Sodium valproate
Sodium valproate is effective in controlling tonic-clonic seizures particularly in primary generalised epilepsy. It is the drug of choice for primary generalised seizures, generalised absences and myoclonic seizures.
Liver function, platelet count, bleeding time/ coagulation and full blood count should be tested before starting treatment (and before surgery also) and then periodically during the first six months of treatment. Severe liver damage, including hepatic failure, is a very rarely reported adverse effect of sodium valproate. Those most at risk are children under the age of three years and those with severe seizure disorders, organic brain disease, or congenital metabolic or degenerative disease associated with mental retardation — especially when using multiple AEDs. The likelihood of severe liver damage is significantly reduced after the age of three years and progressively decreases with age. Patients and carers should be educated to report urgently any signs or symptoms suggesting pancreatic, blood or hepatic disorders (eg, jaundice, malaise, anorexia, lethargy, drowsiness, vomiting and abdominal pain) so valproate can be withdrawn and possible causes can be investigated without delay.
For adults the oral starting dose is usually 600mg daily in two divided doses, increasing by 200mg/day every three days up to a maximum of 2,500mg/day, until adequate control is achieved. A modified-release formulation (Epilim Chrono) allows once-daily dosing, which may improve treatment adherence. Valproate is highly bound to plasma proteins — when used concomitantly with other highly protein-bound drugs, eg, aspirin, free valproic acid levels can increase (hence the valproate dose may need to be reduced to prevent toxicity).
Side effects include gastrointestinal pain, tremor, ataxia, sedation, hair loss, increased appetite and weight gain (>10% body weight).
Phenytoin
Phenytoin is effective for treating tonic-clonic and partial seizures. The medicine shows non-linear pharmacokinetics — ie, small increases in phenytoin dose can produce large increases in plasma drug levels, which increases the risk of toxicity. Plasma drug monitoring is therefore highly valuable to guide dose adjustments.
Patients and carers should be educated on the importance of reporting any fever, sore throat, rash, mouth ulcers, bruising or bleeding immediately, since these may indicate adverse effects associated with blood or skin disorders.
The phenytoin dose should be individualised because there can be wide inter-patient variability in phenytoin serum levels with equivalent dosage. It should be initiated at a low dose, increased in gradual increments determined by plasma drug levels. The dose should be increased until seizure control is achieved or until side effects limit further increases.
Side effects include hirsutism, gum hypertrophy and aggravation of acne. Phenytoin treatment is also associated with SJS and should be discontinued if rash appears.
Phenobarbital and primidone
Phenobarbital is effective against tonic-clonic and partial seizures. It is now generally considered as second-line therapy because of its side effects, which include sedation, agitation, depression, behavioural changes and hyperkinesia (in children), as well as the risk of withdrawal seizures. Primidone has similar uses and side effect profile as phenobarbital (which is one of the active metabolites of primidone).
Newer AEDs
ll “newer” antiepileptic drugs were initially licensed as adjuncts to the established older agents in combination therapy. Although still referred to as “new” most of these drugs have been marketed in the UK for a considerable length of time (see Box). In general, the newer AEDs have simpler pharmacokinetics, are less likely to interact with other medicines, have more favourable side effect profiles and require less laboratory monitoring than the older AEDs.
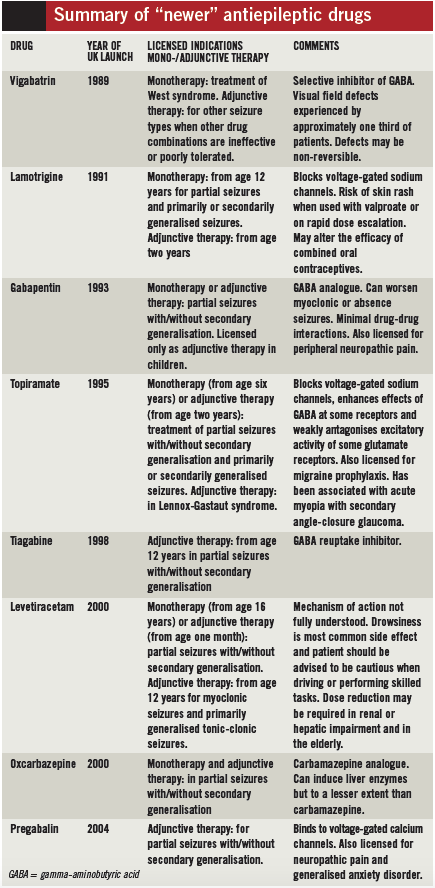
A recent report looking at the uptake of the 2004 NICE guidelines states that in the year leading up to March 2008 the rate of prescribing of newer antiepileptic drugs overall, and as a proportion of all antiepileptic drugs, increased and that these medicines accounted for 39% of AEDs dispensed and 65% of the total cost. A partial update of the clinical guideline is expected in 2011.
“Third-generation” AEDs
Lacosamide is licensed for adjunctive therapy in partial seizures with or without secondary generalisation. Rufinamide is licensed for the adjunctive treatment of Lennox-Gastaut syndrome. There is a risk of severe hypersensitivity syndrome when rufinamide is started, especially in children. Zonisamide is licensed as adjunctive treatment for refractory partial seizures with or without secondary generalisation.
Other AEDs
Benzodiazepines clonazepam and clobazam are licensed for the treatment of tonic-clonic or partial seizures and adjunctive therapy for epilepsy, respectively. However, their usefulness is restricted by sedative side effects and a decrease in efficacy when used continuously long term. On the other hand, benzodiazepines are extremely valuable in the management of status epilepticus.
Comparison of treatment options
The “Standard and newer antiepileptic drugs” (SANAD) study was sponsored by the National Institute for Health Research’s Health Technology Assessment Programme.
This multicentre, non-blind, randomised, controlled study in hospital-based outpatient clinics aimed to compared the longer-term clinical outcomes and costeffectiveness of newer and older AEDs.
The study consisted of two arms: arm A (1,721 subjects recruited) assessed carbamazepine, as the standard treatment, against lamotrigine, oxcarbazepine, gabapentin and topiramate in the treatment of partial epilepsy;9 arm B (716 subjects recruited) assessed the treatment of generalised and unclassifiable epilepsy with sodium valproate, as the standard therapy, against lamotrigine and topiramate as newer agents.10 The two primary outcomes assessed were time to treatment failure and time to 12-month remission.
In arm A, lamotrigine outperformed the other agents in time to treatment failure with the least favourable outcomes for gabapentin and topiramate. Carbamazepine was found to be better than the other agents in the time to 12-month remission measure. In arm B, for the time to treatment failure measure, sodium valproate was found to be better than topiramate, and no significant difference was observed between sodium valproate and lamotrigine. With regard to time to 12-month remission, sodium valproate was found to be better than lamotrigine; no significant difference was found between sodium valproate and topiramate. Although the SANAD research has some methodological weaknesses that have been discussed in many commentaries, it is a large study of substantial duration that has attempted to provide further evidence to guide treatment choice in epilepsy.
Failed response and treatment resistance
When monotherapy with a first-line AED has failed, because of adverse effects or continued seizures, monotherapy with the second-line agent should be tried under the guidance of an epilepsy specialist. Extreme caution is required to limit the breakthrough of seizures when changing from one AED to another. The second drug should be started and an adequate dose established before the first drug is slowly withdrawn.
When treatment for newly diagnosed epilepsy has failed, clinicians should consider the following possibilities:
- An incorrect diagnosis
- An inappropriate choice of treatment for the epilepsy syndrome
- Non-adherence to treatment
- Interaction with other medicines, which might be affecting plasma levels
- An underlying cerebral neoplasm
- Covert drug or alcohol misuse
If treatment has failed with monotherapy, combination treatment with two or at most three AEDs may be necessary. The choice of combinations should take into account the possible increased risk of drug interactions and adverse effects. The patient should be established on the regimen that provides the best balance between tolerability and control of seizures.
If seizure control is not attained with adequate dosing of two AEDs and all other causes of treatment failure have been ruled out, the individual may be suffering from drug-resistant epilepsy. Once drug resistance is determined, tertiary referral is recommended at an early stage since neurosurgery could be an option.
Therapeutic drug monitoring6,7
Routine monitoring of AED blood levels is not recommended and should only be done if clinically indicated. There are clinically useful doseresponse and dose-toxicity relationships for carbamazepine and phenytoin but not for sodium valproate or the newer AEDs. Plasma drug monitoring may be useful for the following:
- Assessing medicines adherence
- Assessing drug toxicity
- Adjusting phenytoin doses — the drug’s pharmacokinetic profile often makes accurate dose adjustment difficult without assessing levels
- Managing patients with specific clinical conditions, eg, during pregnancy, organ failure or status epilepticus
- Managing pharmacokinetic interactions
Different preparations and formulations of AEDs are not necessarily bioequivalent. It is advised to exercise caution when a change in formulation is necessary and use plasma drug monitoring to guide dosing where applicable.
Carbamazepine plasma concentration reaches steady state within about one to two weeks, depending on auto-induction (where the drug induces an increase in its own metabolism). Hence, plasma levels should not be requested less than two weeks after starting treatment or changing the dose, unless toxicity is suspected. Blood should be taken pre-dose and the normal therapeutic range (trough) is 4–12mg/L.
Phenytoin is extensively metabolised by the liver and is highly protein bound. Blood should be taken pre-dose and the normal therapeutic range (trough) is 10–20mg/L.
Special populations
Older patients
The rate and intensity of AED adverse effects tend to be greater in older patients (>65 years). This combined with the high likelihood of polypharmacy can make treatment choice difficult. Decisions should take into account medicines’ side effect profiles and their potential for drug interactions.
Women of childbearing potential
Contraception
Women of childbearing age should be advised of the need for contraception while taking AEDs (see “Pregnancy” section, below). They should also be informed that enzyme-inducing AEDs such as carbamazepine, oxcarbazepine, phenobarbital, topiramate and phenytoin can lower the efficacy of both the combined and progesterone-only oral contraceptive pills, hence alternative contraceptive methods should be discussed and advised where appropriate. NICE provides detailed guidance on contraception in this setting.
Pregnancy
The overall rate of minor and major birth defects seen in offspring born to mothers with epilepsy receiving a single AED is two to three times higher than the rate reported in the general population (approximately 2–3%). Data suggest that the risk may be greater with valproate than with carbamazepine or lamotrigine.
The most common major malformations associated with AEDs include neural tube defects (spina bifida, anencephaly), orofacial defects, congenital heart abnormalities and hypospadias.
Summaries of product characteristics for valproate products recommend that women of childbearing potential should not be started on sodium valproate without specialist advice. When a patient is planning to become pregnant the risks and benefits of continuing valproate treatment throughout pregnancy should be borne in mind and discussed with her. Folate supplementation (folic acid 5mg/day) before pregnancy has been shown to reduce the incidence of neural tube defects and should be offered to all women of childbearing potential who are taking AEDs. It should be continued until the end of the first trimester. If a woman on maintenance valproate treatment becomes pregnant (unplanned), NICE says consideration should be given to stopping the valproate.
If valproate is to be continued during pregnancy:
- Monotherapy is preferred
- Folic acid 5mg/day supplementation should be prescribed
- The lowest effective dose should be used (£1g/day since the incidence of neural tube defects, notably spina bifida, increases above this dose
- Peaks in valproate levels should be avoided by reducing the dosing interval or using slow-release formulations while reducing the dosing interval (eg, use Epilim Chrono rather than Epilim entericcoated tablets; give as 400mg twice a day rather than 800mg once a day)
There are limited data on the safety of the newer AEDs in pregnancy.
Breastfeeding
The benefits of breastfeeding should be weighed against the possibility of adverse effects occurring in the infant.
Valproate concentrations in breast milk are low with a concentration of 1–10% of total maternal serum levels; breastfeeding would therefore appear to be relatively safe although haematological effects have been reported in a small number of infants. Breastfed infants should be monitored closely for liver and platelet changes.
Carbamazepine concentrations in breast milk have been shown to be low. Breastfed infants should be observed for possible adverse effects (eg, excessive somnolence, allergic skin reaction).
Conversely, lamotrigine breast milk concentrations are high. Lamotrigine should be avoided for women who are breastfeeding because of the risk of SJS in the infant.
Children and adolescents
Although treatment choices for children and adolescents are guided by similar principles as for adults, there are some important differences: there are a greater number of causes of epilepsy and epileptic syndromes in children; refractory epilepsy in children is usually generalised epilepsy; the syndrome may vary with the age of the child; and the potential impact on social, behavioural and educational outcomes may be profound in children.
Cognitive and behavioural side effects of AEDs appear more common in children and occurrence of these should prompt a review of therapy. The cognitive effects of AEDs can be difficult to distinguish from the underlying illness. Epilepsy itself may impact on cognition (especially memory) in some children.
Up to 50% of children with epilepsy require additional support in school. Furthermore, the prevalence of epilepsy is high in children with learning difficulties and cerebral palsy. When a child has epilepsy and learning difficulties, he or she is three times more likely to be diagnosed with autism by the age of 10 years.7
The interactions of AEDs with hormonal contraceptives and teratogenic risks should be considered when selecting medicines for adolescent girls.
Many AEDs are not licensed below a particular age and, moreover, altering existing formulations for ease of administration often constitutes unlicensed use. Unlicensed and “off-label” use should be guided by informed choice as detailed in a policy statement issued in 2000 by the Standing Committee of Medicines (a joint committee of the Royal College of Paediatrics and Child Health and the Neonatal and Paediatric Pharmacists Group).
Discontinuing treatment
Clinicians should discuss the possibility of discontinuing AED treatment in adults who have been seizure-free for at least two years. The decision to discontinue treatment must be made jointly between the patient/carer and a specialist and be based on the patient’s risk of seizures, lifestyle, prognosis and seizure syndrome.
Few studies have reported on AED discontinuation in adults who were seizure-free for at least two years. One prospective multicentre randomised study (1,013 patients) looked at continued AED treatment versus slow withdrawal of treatment over six months; seizure relapse occurred for 22% of patients who continued treatment compared with 41% of the withdrawal group.11 Similar results were observed in another study of 330 patients.12
A specialist should manage the planned withdrawal of AEDs. Only one drug should be withdrawn at a time. AED treatment should normally be stopped gradually over at least two to three months, preferably longer, and six months or longer for barbiturates and benzodiazepines.
In the event of a medical emergency, eg, toxicity or hypersensitivity reaction, abrupt discontinuation of the AED might be necessary.
Management of status epilepticus
Status epilepticus may present as convulsive or nonconvulsive seizures, although non-convulsive status epilepticus is uncommon and management is less urgent. Generalised tonic-clonic status epilepticus is a medical emergency associated with significant morbidity and mortality, necessitating rapid diagnosis and emergency treatment in secondary care. Local protocols for the management of status epilepticus exist in many trusts.
Parenteral thiamine and pyridoxine are also used in the management of status epilepticus where the underlying cause is alcohol misuse or genetic pyridoxine deficiency.
For children, the Status Epilepticus Working Party of the British Paediatric Neurology Association produced a consensus guideline in 2000.13 An algorithm from this guideline is reproduced in appendices to the NICE and SIGN epilepsy guidelines.
Initial management
Pre-hospital care includes securing the airway, giving oxygen, assessing cardiac and respiratory function and (where applicable) correcting hypoglycaemia. If resuscitation facilities are not immediately available initial treatment is with diazepam or buccal midazolam (unlicensed use).
Diazepam solution is given rectally. The usual dose for adults is 10–20mg (10mg for the elderly), and the dose can be repeated once after 15 minutes.
For children and neonates, the dosing of rectal diazepam solution ranges from 1.25mg to 10mg depending on the age of the child. A second dose can be repeated after five minutes. For children aged over 10 years and adults the dose of buccal midazolam is 10mg, repeated if necessary after 10 minutes. Dosing varies with age for younger children and neonates.
In hospital, convulsive status epilepticus is treated with intravenous lorazepam or clonazepam repeated after 10 minutes if seizures recur. Formulations of diazepam other than rectal solution are not recommended because of a high risk of thrombophlebitis with intravenous use and the slow absorption from suppositories or the intramuscular route.
Failed response
If seizures fail to respond within 30 minutes or recur, phenytoin sodium can be administered by slow intravenous infusion. Fosphenytoin is a prodrug of phenytoin that can be given more rapidly and is associated with fewer injection site reactions than phenytoin. Both agents require electrocardiogram monitoring due to the risk of severe cardiovascular reactions with intravenous administration. Intramuscular phenytoin administration is too slow and erratic to be appropriate.
Phenobarbital sodium may also be used intravenously to treat status epilepticus. Rectally administered paraldehyde has a lower risk of causing respiratory depression and can be a useful alternative where facilities for resuscitation are poor.
Refractory status epilepticus
Refractory status epilepticus in adults (seizures fail to respond within 60 minutes of treatment with phenytoin sodium, fosphenytoin or phenobarbital sodium) requires initiation of general anaesthesia using midazolam, thiopental or propofol (unlicensed use). Full intensive care support should be available.
References
- Elger CE, Schmidt D. Modern management of epilepsy: a practical approach. Epilepsy & Behavior 2008;12:501–39.
- Stein MA, Kanner AM. Management of newly diagnosed epilepsy: a practical guide to monotherapy. Drugs 2009;69:199–222.
- National Institute for Health and Clinical Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. October 2004. www.nice.org.uk/cg20 (accessed 22 February 2010).
- National Institute for Health and Clinical Excellence. Newer drugs for epilepsy in adults. March 2004. www.nice.org.uk/ta76 (accessed 22 February 2010).
- National Institute for Health and Clinical Excellence. Newer drugs for epilepsy in children. April 2004. www.nice.org.uk/ta79 (accessed 22 February 2010).
- Scottish Intercollegiate Guidelines Network. Diagnosis and management of epilepsy in adults. April 2003 (updated October 2005). www.sign.ac.uk/ guidelines/fulltext/70/index.html (accessed 22 February 2010).
- Scottish Intercollegiate Guidelines Network. Diagnosis and management of epilepsies in children and young people. March 2005. www.sign.ac.uk/ guidelines/fulltext/81/index.html (accessed 22 February 2010).
- Medicines and Healthcare products Regulatory Agency. Antiepileptics: adverse effects on bone. Drug Safety Update 2009;2(9):2.
- Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1000–15.
- Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1016–26.
- Medical Research Council Antiepileptic Drug Withdrawal Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Lancet 1991;337:1175–80.
- Specchio LM, Tramacere L, Neve AL, et al. Discontinuing antiepileptic drugs in patients who are seizure free on monotherapy. Journal of Neurology, Neurosurgery and Psychiatry 2002;71:22–5.
- Appleton R, Choonara I, Martland T, et al. The treatment of convulsive status epilepticus in children. Archives of Disease in Childhood 2000;83:415–9.


