This content was published in 2011. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Key points
- Patients experiencing a “first fit” should be referred within 14 days for investigation.
- Seizures lasting more than five minutes require medical assistance.
- With optimal therapy, epilepsy is controlled in about 70 per cent of patients.
- Choice of agent is based on epilepsy syndrome, seizure type and individual patient factors. (Some antiepileptic drugs can worsen seizure frequency of some epilepsy types.)
- Changing the formulation or brand of AED is not recommended because of variation in bioavailability or pharmacokinetic profiles.
- Where effective, monotherapy should be continued until the patient remains seizure free for at least two years, after which, controlled withdrawal may be considered.
- Antihistamines and evening primrose oil can make seizures more likely.
- Vitamin D supplements may be considered for at risk patients on long-term treatment with primidone, phenytoin, carbamazepine, phenobarbital or sodium valproate
To suffer from epilepsy means to have an enduring predisposition to epileptic seizure unprovoked by any immediately identifiable cause.1 One in 20 people will have a single epileptic seizure in their lifetime. This does not necessarily confer a diagnosis of epilepsy2 — the seizure could be due to transient noxious stimuli (eg, in hypoglycaemia or hypoxia). In addition, other conditions, such as syncope, cardiac arrhythmias, panic attacks and pseudoseizures, can present similarly and must be considered.
A diagnosis of epilepsy can have a significant impact on an individual’s life, not least in terms of restrictions on driving, life insurance premiums and the burden of longterm use of antiepileptic medicines, many of which come with unpleasant or potentially serious adverse effects. A diagnosis should, therefore, not be made lightly and patients experiencing a “first fit” should be referred within 14 days for electroencephalography (EEG), magnetic resonance imaging (MRI) and computed tomography (CT), and a consultation with a neurologist.3
These investigations can also aid in differentiating the specific epileptic syndrome and thus guide likely effective treatment (see later).
Neurotransmitter imbalance
Epileptic seizures are characterised by episodic high frequency neuronal discharge due to an imbalance in excitatory and inhibitory neurotransmitter release. An excess of excitatory neurotransmitter (eg, glutamate) or deficit of inhibitory neurotransmitter (eg, gamma-aminobutyric acid) leads to postsynaptic neuronal activation and overfiring of the action potential (see Figure 1).
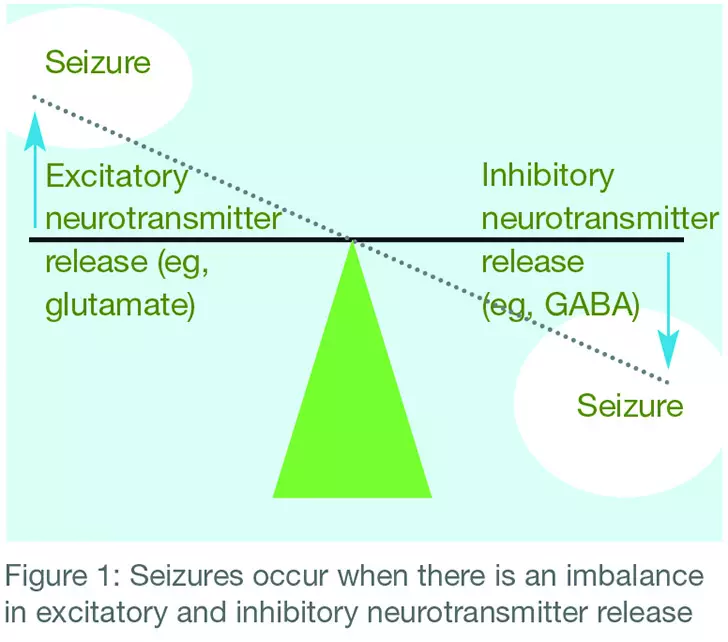
in excitatory and inhibitory neurotransmitter release
The area of the brain where the high frequency firing of neurones originates is known as the epileptic focus and this, coupled with the pattern of spread of the impulse discharge to other areas of the brain, confers the particular symptoms that characterise an individual’s syndrome.
Reflect
- How is a diagnosis of epilepsy made?
- How do antiepileptic drugs work?
- How effective is adopting a ketogenic diet to prevent epilepsy?
Before reading on, think about how this article may help you to do your job better.
Seizure types and syndromes
Where part of one side of the brain is affected, the seizure is “partial”. Partial seizures can be described as simple (the person is aware of what is happening) or complex (the person is unaware or confused).
Generalised seizures occur if the whole brain, throughout both hemispheres, becomes involved. These seizures can also be described as different types but in all of them the person will not be conscious of the seizure. Examples of generalised seizure types are:
- Tonic-clonic (or “grand mal”)
- Absence (or “petit mal”)
- Myoclonic
- Tonic
- Atonic
The action needed when someone has a seizure depends on its type. For example, a simple partial seizure might involve twitching in a limb or a sudden feeling of fear or joy, and the person may simply require gentle reassurance. However, with tonic-clonic seizures, more assistance will be needed.
In the tonic phase the person goes stiff, which usually means they fall down. In the clonic phase they have convulsions.
Breathing can be affected and they may go pale or blue. Injuries that can occur in patients with seizures include tongue lacerations, shoulder dislocations and head trauma. Panel 1 lists general action to take if someone has a seizure.

Epilepsy itself is often also classified into syndromes, depending on the group of symptoms the person has (eg, seizure type) and other factors, such as the age at which seizures begin. In fact, epilepsy describes around 30 different syndromes collectively affecting nearly half a million of the UK population.4 These include, for example, childhood absence epilepsy, juvenile absence epilepsy, benign epilepsy, continuous spike wave of slow sleep and Lennox-Gastaut syndrome.
If a seizure lasts for more than 30 minutes or a person has a series of seizures without regaining consciousness, this is termed status epilepticus. Generalised convulsive status epilepticus occurs when abnormal excessive cortical electrical activity leads to a continuing seizure with motor activity (convulsive movements). This seizure and its treatment are discussed further in Panel 2.

Causes and triggers
About a third of epilepsy cases have an anatomically identifiable cause. The likelihood of such a cause increases with age. Within this group, 15 per cent of cases are linked to cerebrovascular disease and 6 per cent to cerebral trauma. Other causes include perinatal brain injury secondary to trauma or hypoxia, cortical or vascular malformation, cerebral abscess and brain surgery.
The remaining two thirds of cases are idiopathic, and are more common in the young; often there is a genetic predisposition to a lower seizure threshold. They often involve complex congenital abnormalities in the balance of neurotransmitter release or synaptic function.
Some people find that their seizures are triggered by certain stimuli, including alcohol, lack of sleep, stress, flashing lights, illness (eg, fever), menstrual cycle, fasting, medicines, supplements and drugs. For example, antidepressants, antihistamines, evening primrose oil and cocaine can make seizures more likely.
Antiepileptic drug therapy
Mortality in newly diagnosed epilepsy is largely attributable to an underlying disease causing the seizure (eg, vascular disease, tumour). In chronic epilepsy, however, the main cause of mortality is a seizure, known as sudden unexpected death in epilepsy (SUDEP). Effective treatment to prevent seizures is, therefore, important.
Following occurrence of two unprovoked epileptic seizures leading to a diagnosis of epilepsy, and ideally the identification of the specific seizure type and epilepsy syndrome, treatment with antiepileptic drugs (AEDs) is usually initiated. The goal is to control seizures rapidly, using the lowest effective dose of, ideally, a single agent, without intolerable adverse effects or toxicity.
About a third of epilepsy sufferers willexperience less than one seizure a year, one third have between one and 12 seizures per year and the remainder have more than one seizure per month.6 The first-known antiepileptic to demonstrate antiseizure activity was phenobarbitone (1910) and until the introduction of phenytoin in 1940, this and similar drugs, such as primidone, were the only agents available. There are now over 20 UK licensed AEDs available but the early therapies retain a place in management.
With optimal therapy, epilepsy is controlled in about 70 per cent of patients. Those remaining seizure free for five years, either on or off treatment, are considered to be in remission.7 Until this point, they will require yearly reviews.
The remainder are considered to have refractory epilepsy and may continue to have frequent seizures despite using multiple AEDs at maximal tolerated doses.
The pharmacology of the AEDs centre around reducing the excitation of neurones.
Glutamate and GABA receptors and ion channels in the postsynaptic membrane are all drug targets (see Panel 3).

In most circumstances, treatment with an AED should be initiated by a specialist.
Prescribing can usually be continued in primary care in accordance with an individualised treatment plan.
Choice of agent will be based on epilepsy syndrome, seizure type and individual patient factors, and should be made jointly between the specialist and the patient (and carers).
For example, carbamazepine is usually used first-line for generalised tonic-clonic, focal or benign epilepsies whereas ethosuximide is usually used first-line for childhood absence epilepsy and continuous spike wave of slow sleep. Agent choice is an important consideration because some AEDs can worsen seizure frequency of some epilepsy types. For example, tiagabine and vigabatrin should be avoided in patients who have tonic-clonic seizures.3
Most AEDs, are initiated according to the old adage “start low, go slow” and titrated according to individual agent recommendations in the British National Formulary or on the recommendation of a specialist. An exception to this rule is phenytoin which, due to its complex kinetic profile, is usually prescribed at full maintenance dose following a loading dose in secondary care.
Patient care
Most patients will have received some medication counselling in secondary care on treatment initiation. However, this is often at a time when they are being bombarded with new information. Collecting a repeat prescription is the ideal opportunity for pharmacists to discuss how patients are getting on with their AED. Such a discussion should always include asking about adherence with medication, any recent seizure activity and any adverse effects.
It should be remembered that changing the formulation or brand of AED is not recommended because different products can vary in bioavailability or pharmacokinetic profiles and switching can increase the potential for reduced efficacy or excessive side effects.
Adverse effects of AEDs can range from transient fatigue (as with clobazam and clonazepam) to skin rash (rarely, Stevens-Johnson syndrome) and blood dyscrasias, and pharmacists should be able to recommend action to be taken, which might include hospital admission, dose reduction, blood tests, folic acid (to treat anaemia) and exercising good dental hygiene (for gingival hypertrophy; see part 2 of this article for more details). Dose reduction can sometimes be transient, reducing to a previous dose and then titrating more slowly.
Patients who experience intolerable adverse effects that do not resolve on dose reduction (or where reduction brings about recurrence of seizures) and those who continue to have seizures despite maximal AED doses may be considered for an alternative AED. The second AED (which may be an alternative first-line or a second-line drug) should be started and built up to an adequate or maximum tolerated dose before slowly tapering the first drug. Interactions between the two should be considered (see below).
Where effective, AED monotherapy should be continued until the patient remains seizure free for at least two years, after which, controlled withdrawal may be considered with agreement of the patient (or carer) and the specialist.
Note that tolerance of clobazam and clonazepam can develop with prolonged use and drug choice may need to be reviewed.
The Commission on Human Medicines has recommended that vitamin D supplements be considered for those on longterm treatment with primidone, phenytoin, carbamazepine, phenobarbital or sodium valproate, and who have other risk factors for osteopenia, osteoporosis and increased fractures (eg, inadequate sun exposure) because data suggest that long-term use of these AEDs is associated with decreased bone mineral density.8
Combination therapy considerations
Although monotherapy remains the mainstay of epilepsy treatment, combination treatment with two or more AEDs may be necessary in some difficult to treat patients. It is here that pharmacists’ knowledge of drug interactions and its clinical application can be invaluable.
Enzyme induction
Primarily it is the older AEDs which cause the most clinically significant interactions. Carbamazepine, phenytoin and phenobarbital are all inducers of both the cytochro (notably CYP1A2, CYP2C9, CYP2C19 and CYP3A4) and glucuronyl transferase (GT) enzyme systems. This leads to faster induction of drugs that are substrates at these sites, usually translating to reduction of the serum concentration. Many other AEDs are affected by this induction, especially lamotrigine (plasma concentration reduced by over 50 per cent) and valproate (reduced by 50–75 per cent).
Enzyme inhibition
Conversely, sodium valproate is an enzyme inhibitor. It causes an elevation in plasma concentrations of the active metabolite of carbamazepine, and of lamotrigine, phenobarbital and phenytoin when these agents are co-prescribed.
The interaction with lamotrigine is particularly significant and can lead to as much as a two-fold rise in lamotrigine serum concentration. Lamotrigine-induced skin rashes are concentration-dependent so when starting lamotrigine for a valproate-treated patient, low starting doses (eg, 25mg on alternate days) should be used, and the drug titrated more slowly to lower target doses. If valproate is withdrawn there may be a rebound reduction in lamotrigine levels and patients should be closely monitored.
Enzyme induction and inhibition by these AEDs should also be considered when coprescribing any non-epilepsy drugs.
In general, the newer AEDs are less likely to be implicated in causing clinically significant drug interactions. However, they are often substrates for the affected enzymes and subject to interactions mediated by enzyme induction or inhibition caused by the older and frequently used AEDs. Levels of levetiracetam, gabapentin and vigabatrin are not affected to any clinically significant exten by co-medication with other AEDs.
Refractory epilepsy
For patients refractory to pharmacological treatment of epilepsy, referral should be made from secondary to tertiary care, where neurologists will review patients’ suitability for alternative management strategies such as resective surgery or vagus nerve stimulation. For children, the ketogenic diet may be recommended at this stage.
Ketogenic diets
The ketogenic diet is an increasingly popular non-drug treatment option for children with refractory epilepsy. Developed in the early 20th century, it comprises high fat, adequate protein and low carbohydrate nutrition.
This balance of nutrients, carefully worked out by a specialist dietitian, induces a state of ketosis and the resulting biochemical changes associated with prolonged fasting have been associated with treating seizures in children.
It is not fully understood how inducing ketosis controls seizures, but various theories have been suggested, including that:
- Ketone bodies have a direct stabilising effect on the central nervous system
- Ketosis resulting in acidosis modifies the seizure threshold
- Fluid and electrolyte changes lead to a reduction in seizures
- High lipid levels produced by the diet have an antiseizure effect
- Neuroprotection from free oxygen radicals or prevention of apoptosis are antiepileptic
A systematic review of the available literature suggests that approximately half of the children with refractory epilepsy should have a clinically meaningful improvement in seizures (greater than 50 per cent reduction) with introduction of the ketogenic diet.9 If the diet is effective it typically works within the first two weeks. Improvements in sleep quality, cognition and behaviour have also been attributed to the ketogenic diet.
There are limited studies of the ketogenic diet in adults and therefore little is known regarding the efficacy or potential adverse effects in this population. Studies involving a less restrictive modified Atkins diet are underway and initial results are promising.
Adverse effects of the ketogenic diet
Early onset complications (usually occurring within four weeks of starting the diet) can include dehydration, gastrointestinal disturbances, hypertriglyceridaemia, transient hyperuricaemia and symptomatic hypoglycaemia. Later onset complications can include osteopenia, renal stones, cardiomyopathy, hypocarnitinaemia and iron deficiency anaemia.
Most complications are transient and can be easily managed with conservative treatments. Oral potassium citrate supplements can be used to prevent renal stones. Some centres give them to all patients whereas others only use them in patients considered to be high risk, for example, those on topiramate (renal calculi are a side effect), those with a personal or family history of renal stones, and those with poor fluid intake.
Special groups
It is important to recognise that people with epilepsy may have co-morbidities. For example, children with epilepsy may have attention or learning difficulties. And up to 75 per cent of people with cerebral palsy or postnatal brain injury have epilepsy.
Prescribing and medicines management in children with epilepsy, including special considerations for those on ketogenic diets, is discussed in these Learning & development.
The second part of this CPD article, available online on 18 June 2011, will help pharmacists give advice to women of childbearing age who have epilepsy, and other groups requiring special consideration. Part 2 will also contain references, resources and practice points. “Check your learning” will be available from 18 June.
The citation information is currently available. For more information please contact pharmpress-support@rpharms.com
You might also be interested in…

Epilepsy drugs levetiracetam and zonisamide should not be routinely used first line in the NHS, conclude researchers

No difference in cognitive outcomes in children born to women taking antiseizure medicines
