This content was published in 2011. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.

Hartphotography/Dreamstime.com
Women of child-bearing age
The diagnosis of epilepsy and the use of antiepileptic drugs (AEDs) in women of child-bearing age gives rise to a number of important issues. First, epilepsy and its drug treatment can affect the menstrual cycle and fertility. Second, AEDs are notorious for interacting with numerous other drugs, including hormonal contraceptives, rendering them ineffective. This is a particularly important issue given the risks associated with AEDs in terms of the fetus and potential teratogenicity.
Women of child-bearing age should be counselled on effective contraception and the risks and benefits associated with each method. Panel 4 summarises guidance as a result of the effect of AEDs.3

In addition, all women taking lamotrigine and any oestrogen-based contraceptive should be advised that the hormones can significantly reduce lamotrigine levels, leading to potential loss of seizure control. This is important when starting or stopping the contraceptive because the dose of lamotrigine may need to be adjusted.
Ideally, pregnancy should be planned in women with epilepsy so that any necessary changes to drug therapy can be made and properly assessed before contraception is stopped.
The main AED associated with birth defects and impaired developmental and cognitive outcomes in children is valproate and, for this reason, it should not be prescribed as first-line therapy in women of child-bearing age. Thorough counselling regarding contraception and the risks to a developing fetus must be fully explained to any women for whom using valproate is considered to be the best option. Doses of less than 800mg per day are considered to be associated with less risk than higher doses.
Reflect
- Which antiepileptic drug therapies have the greatest risk of birth defects?
- Should antiepileptic drugs be changed in pregnancy?
- Which antiepileptic drugs can pose a problem in breastfeeding? Before reading on, think about how this article may help you to do your job better.
Pregnant women
Most women with epilepsy who become pregnant will have uneventful pregnancies and give birth to normal babies. However maternal epilepsy and AED treatment both pose a risk for abnormal pregnancy outcome and, therefore, should be managed by specialists in both areas.
Uncontrolled seizures during pregnancy can adversely affect both the mother and unborn baby. For example, it has been suggested that lactic acidosis caused by convulsive seizures may be linked to fetal bradycardia and status epilepticus to interuterine death. The main seizure type thought to be associated with the greatest harm to the fetus is generalised tonic-clonic.
In most cases, seizure control does not deteriorate during pregnancy. In the few cases where seizure frequency does increase, one reason for this is the pharmacokinetic differences in AED handling in pregnant women due to the physiological changes that occur during pregnancy. Concentrations of phenytoin and phenobarbital have been shown to almost halve in late pregnancy and lamotrigine to decrease to 30 per cent of prepregnancy levels. Decreased plasma protein binding can contribute to a decrease in total plasma concentrations of valproate, phenytoin and tiagabine.
Nevertheless, therapeutic drug monitoring is not routinely required during pregnancy unless seizure frequency increases, management of pharmacokinetic interactions is required, or if non-adherence, organ failure or drug toxicity is suspected.
Careful interpretation of plasma levels is required and free drug levels should be taken where possible for highly protein bound drugs.
This risk of having seizures is considered to be greater than the risk of using AEDs so continuation of therapy is considered appropriate in most cases — AED therapy aims to reduce both risks to the mother and the fetus.
Use of the older AEDs (phenytoin, carbamazepine, valproate and the barbiturates) during pregnancy has been associated with an increased risk of congenital malformations and, possibly, an adverse effect on fetal growth and psychomotor development. Data show that significantly more children born to women taking these AEDs had congenital malformations compared with the general population. The risks of congenital malformations are also significantly greater for women taking polytherapy.
Although information on the effects of newly licensed antiepileptic drugs on birth defects is sparse, a recently published large cohort study indicates that exposure to newergeneration antiepileptic drugs (lamotrigine, oxcarbazepine, topiramate, gabapentin or levetiracetam) in the first trimester of pregnancy is not associated with an increased risk of major birth defects.10 Of 1,532 babies born to women on these AEDs in Denmark between 1 January 1996 and 30 September 2008, 49 were diagnosed with a major birth defect compared with 19,911 of the 836,263 who were not exposed to an antiepileptic drug (3.2 per cent versus 2.4 per cent; adjusted prevalence odds ratio 0.99; 95 per cent confidence interval 0.72–1.36).
In 1996 the UK Epilepsy and Pregnancy Register was established to obtain and publish information on the frequency of major malformations (eg, heart defect and cleft lip), in children born to women on AEDs. Pharmacists can encourage women with epilepsy to register early in pregnancy (tel 0800 389 1248).
Despite counselling, many pregnancies are often unplanned. The National Institute for Health and Clinical Excellence Guidelines suggest that changing AED regimens during pregnancy may pose more risk than benefit.3
Folic acid
Folic acid has been shown to have a major role in the prevention of neural tube defects. In women with epilepsy, low serum and red blood cell folate levels are associated with an increased incidence of spontaneous abortions and malformations. Despite a lack of clinical trials investigating the role of folic acid in preventing congenital malformations in pregnant women taking AEDs, clinical experience suggests higher than normal dose (5mg) is recommended for all women on AEDs trying to get pregnant and throughout at least the first trimester.
Breastfeeding
The benefits of breastfeeding are well recognised and this applies to infants born to mothers taking AEDs. If a woman has been taking a particular drug during pregnancy, there seems to be little risk in breastfeeding the infant because the amount in the breast milk is likely to be relatively small. The older AEDs are all excreted in breast milk and for most of these drugs, continuation while breastfeeding is recommended. The main established drug that can potentially cause a problem is phenobarbital. Due to slower elimination in the nursing infant, accumulation can occur and the child should be observed for signs of toxicity, such as sedation.
There is less information available regarding the newer antiepileptics in breastfeeding. Lamotrigine is an example of one of the newer agents where there is conflicting information. Although there are no reports of adverse effects in infants, relatively high concentrations (30–35 per cent) of the maternal dose have been reported in breast milk. Following a thorough assessment of both the risks and benefits it is advised that if lamotrigine is to be continued the infant should be monitored for signs of toxicity (apnoea, rash, drowsiness, poor feeding) and serum levels should be taken if these develop.
Women wishing to breast feed should be encouraged to do so but each case should be discussed with healthcare professionals and the risks and benefits explained so that they can make informed decisions.
Patients with learning disabilities
It is estimated that 15 per cent of people with mild learning disabilities and 30 per cent of those with severe learning disabilities have epilepsy. These conditions commonly coexist and most often develop in childhood; the earlier the seizure onset, the more severe the learning disability is likely to be. It is important that this group of patients are particularly supported in decisions involving their care, especially if they are lacking the mental capacity to make their own decisions. An advocate should be sought who places the individual’s best interests foremost. Individuals should be encouraged to take an active part in developing a personalised care plan for treating their epilepsy.
All healthcare professionals should recognise that extra time may be required to tailor drug regimens to the individual’s needs and to reduce the risk of discontinuation and intolerable side effects. Particular care should be paid to the possibility of adverse cognitive and behavioural effects (eg, impaired concentration, hearing disorders, irritability and aggression) of AED therapy.
Summary
Pharmacists have the best pharmacological and pharmacokinetic education at undergraduate level of any of the healthcare professions. Those who maintain and develop this knowledge can play a significant role in the management of patients with AEDs. Expertise in advising on drug interactions, the ability to interpret therapeutic drug monitoring to ensure effective dosing with minimal toxicity, and skills in negotiating a patient’s agenda to improve adherence mean that pharmacists specialising in epilepsy may find themselves an invaluable member of the multidisciplinary team.
Key points
- Women of child-bearing age and who have epilepsy should be counselled on effective contraception. Adjustments may be needed depending on the antiepileptic drug used.
- Maternal epilepsy and antiepileptic drug treatment both pose a risk for abnormal pregnancy outcome.
- Pregnancy women with epilepsy should be encouraged to join the UK Epilepsy and Pregnancy Register.
- Patients with learning difficulties should be supported in decisions involving their care.
Practice points
Reading is only one way to undertake CPD and the regulator will expect to see various approaches in a pharmacist’s CPD portfolio.
- Carry out a medicines use review with a patient with epilepsy. Discuss the the patient’s AED history, including previous unsuccessful therapies and any adverse effects experienced. Reflect on the appropriateness of the medication history.
- Discuss with a colleague how you would manage the first-line AED therapy for a patient from one of the special groups.
- Review your AED counselling points to ensure that you can explain what to do should a patient suffer from an adverse effect of a drug.
Consider making this activity one of your nine CPD entries this year.
Resources
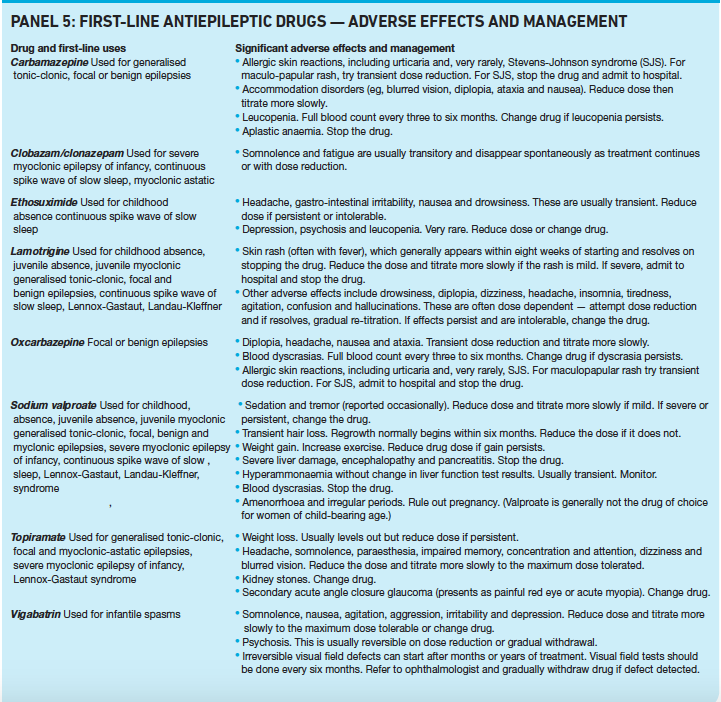
- Panel 5 summarises the first-line antiepileptics and their adverse effects. Pharmacists may find it useful for determining the severity of an adverse effect and recommending action to be taken. However, this list is not exhaustive and should be used in conjunction with up-to-date clinical guidelines and summaries of product characteristics.
- Quick guidance from NICE, “The epilepsies: diagnosis and management of the epilepsies in adults in primary and secondary care”, has a useful table recommending antiepileptics for use (first-line, second-line and further) in different types of epilepsy. New NICE guidance on epilepsy is expected in January 2012.
- The Epilepsy Society website describes different types of seizure and what action is needed with each.
References
- Fisher RS, Van Emde Boas W, Blume W, Elger C, Genton P, Lee P et al. 2005. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470–2.
- The National Society for Epilepsy. Fact Sheet 2. Available at www.epilepsysociety.org.uk (accessed on 14 April 2011).
- National Institute for Health and Clinical Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. CG20; 2004. Available at www.nice.org (accessed on 23 May 2011).
- Joint Epilepsy Council. Epilepsy prevalence, incidence and other statistics. 2005 Available at http://jointepilepsycouncil.org.uk (accessed on 15 April 2011).
- Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia 1999;40:120–2.
- Brown S, Betts T, Crawford P, Hall B, Shorvon S, Wallace S. Epilepsy needs revisited: a revised epilepsy needs document for the UK. Seizure 1998;7:435–46.
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346:140–4.
- Medicines and Healthcare products Regulatory Agency & Commission on Human Medicines. Antiepileptics: adverse effects on bone. Drug Safety Update; April 2009.
- Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics 2000;105;e46.
- Mølgaard-Nielsen D, Hviid A. Newer generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002.
CPD articles are commissioned by The Pharmaceutical Journal and are not peer reviewed.


