Science Photo Library
Tuberculosis (TB) continues to pose a significant global health challenge and is one of the leading causes of mortality worldwide from an infectious disease, with an estimated 1.8 million TB deaths attributable in 2015[1]
. TB is a transmissible infection caused by the bacteria of the Mycobacterium tuberculosis complex. It may affect the lungs (pulmonary TB) or other body sites (extrapulmonary TB). Haematogenous dissemination of TB to multiple organs is referred to as miliary TB. Infection occurs upon inhalation of M. tuberculosis organisms and can result in clinically latent infection or active disease.
Latent TB is a state in which the bacteria remain viable but are contained by the immune system, so that there is no clinical manifestation of TB disease[2]
. Active TB occurs when the immune system fails to contain or eliminate the bacteria, resulting in clinical disease. Symptoms of active TB are variable and depend on the site of infection.
Pulmonary TB is characterised by a persisting cough, sputum production with or without blood, fever, night sweats, fatigue and weight loss[3]
. A large proportion of TB cases are diagnosed presumptively as laboratory facilities may be unavailable beyond sputum smear[1]
. This has implications for selection of appropriate treatment.
Among the many challenges in treatment for TB is the increasing threat of multidrug-resistant TB (MDR TB), which is defined as TB caused by M. tuberculosis isolates that are resistant to both rifampicin and isoniazid[4]
– two of the key drugs active against M. tuberculosis. In 2015, of the estimated 580,000 new cases eligible for anti-MDR TB treatment globally, only 20% received it. This has been driven by health system disparities and failures[1]
– MDR TB thrives in fractured healthcare systems where money and health service provisions are scarce, but the treatment can work if it is employed in the appropriate way with the right support[5]
.
Other contributing factors include the treatment being inherently complex owing to the number of antibiotics and the length of treatment required, the potential for serious adverse effects, the possibility of interactions with other medication and the costs incurred[6]
. A further cause for alarm is the emergence of extensively drug-resistant TB (XDR TB), which is defined as TB caused by M. tuberculosis isolates that are resistant to rifampicin, isoniazid, any fluoroquinolone and at least one of the second-line injectable drugs (capreomycin, kanamycin and amikacin)[1]
.
This article provides an overview of the current treatment regimens for drug-sensitive and drug-resistant TB. Subsequently, it also explores the role of fluoroquinolones in treating TB, discusses the studies evaluating their utility in anti-TB regimens and considers the clinical implications of their use. Finally, discussions relate to areas where fluoroquinolone use requires further research.
Current TB treatment regimens
Drug-sensitive TB
The World Health Organization (WHO) produces regularly updated guidelines on the treatment of drug-sensitive TB[7]
. The treatment of all cases of TB may be divided into two phases: an intensive phase, comprising therapy with isoniazid, rifampicin, pyrazinamide and ethambutol for two months; followed by the continuation phase, with isoniazid and rifampicin for four months. These drugs are all administered orally.
Currently, six months is the shortest recommended length of treatment. To ensure cure at particular sites of disease, this may be increased; for example, 12 months is recommended for TB meningitis and, in some guidance, 9 months is recommended when it affects bones or joints[8]
.
Fixed-dose combination (FDC) regimens are advocated. It has been shown that the efficacy of a four-drug FDC regimen is no worse than a regimen of separately administered drugs[9]
. A FDC is felt to be advantageous as patients cannot be selective about which specific drugs they ingest and it involves taking fewer tablets which, in turn, may promote greater treatment adherence[7]
.
However, these regimens are frequently not used in high-income, low-prevalence settings where supervision of treatment is possible and they are also not available in some high-income settings. Additionally, a recent systematic review found that there was no difference in outcomes between FDC therapy and single-drug formulations[10]
, while a Cochrane review has also reported that the outcomes for patients with pulmonary TB are probably similar when comparing FDC and single-drug formulation therapy[11]
.
The prolonged duration of the course of current anti-TB medication poses a challenge to therapy adherence. Patients may discontinue treatment if they experience an improvement in their clinical state, run out of medication or if they can no longer afford the cost. Hence, socioeconomic factors and patients’ understanding of TB can directly influence treatment adherence[12]
.
The adverse event profile of anti-TB drugs may be intolerable to patients or be severe enough to prohibit further administration of the agent. Additionally, interactions with other drugs may reduce their efficacy or increase their toxicity, rendering them clinically unviable[7]
.
The response to anti-TB therapy and associated adverse effects requires regular monitoring[13]
. Given the risk of hepatitis from isoniazid, rifampicin and pyrazinamide, it is important to assess liver function prior to and during treatment.
Drug-resistant TB
If MDR TB is confirmed, patients should be treated with a regimen tailored to the drug susceptibility testing (DST) results of any isolated organism. WHO and National Institute for Health and Care Excellence (NICE) guidance for treatment of drug-resistant TB was updated in 2016[14],
[15]
. It is important for healthcare professionals to consult with individuals experienced in managing MDR TB to select an appropriate regimen that takes account of patient-specific and bacteria-specific factors.
The precise regimen requires consideration of the following principles[14],
[16]
: at least five effective TB medications should be used during the intensive phase of treatment, including pyrazinamide and four core second-line anti-TB drugs; the intensive phase of treatment should last at least eight months; drugs should be avoided if they are not safe or if they may show cross-resistance (i.e. a phenomenon whereby a mutation in M. tuberculosis to one drug may confer the isolate with resistance to other drugs either in the same or indeed other drug groups); and a greater number of drugs should be used if there is uncertainty about the effectiveness of any of the drugs. Hence, this is a complex area where prolonged and often unpleasant treatment regimens may be needed. WHO issued a conditional recommendation in 2016 regarding the ‘Bangladesh regimen’, which arose from an observational study of more than 500 patients and showed good outcomes when using a modified, shortened treatment regimen for MDR TB[17]
. WHO advised that a shorter MDR TB regimen, lasting between 9 and 12 months, could be employed provided patients meet certain criteria. The regimen comprises 4–6 months of kanamycin, moxifloxacin, prothionamide, clofazimine, pyrazinamide, high-dose isoniazid and ethambutol; followed by 5 months of moxifloxacin, clofazimine, pyrazinamide and ethambutol[18]
.
It should be noted that the cost of TB treatment is highly variable. A recent review has suggested that for each patient with drug-sensitive TB, this can range between US$260 in low-income countries and US$14,660 in high-income countries, while for a patient with MDR TB it is much more expensive, at around US$1,200 in low-income countries and US$83,400 in high-income countries[19]
.
The use of fluoroquinolones in the treatment of TB
History of fluoroquinolones
The first quinolone to be synthesised was nalidixic acid in 1962[20]
. Since then, several others have been developed, including ciprofloxacin and ofloxacin (second generation drugs); levofloxacin (third generation); and moxifloxacin and gatifloxacin (fourth generation)[21]
. Fluoroquinolones inhibit M. tuberculosis DNA gyrase function[22]
, resulting in impaired DNA synthesis and subsequent cell death. Their pharmacokinetics are such that when taken by mouth, they can generally achieve serum concentrations greater than the minimum inhibitory concentration of M. tuberculosis
[23],
[24]
.
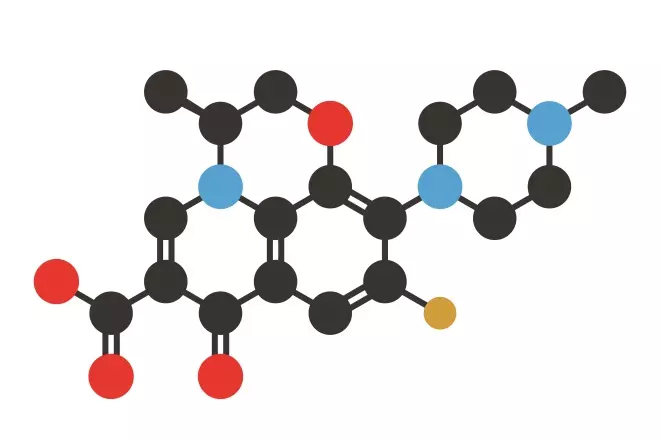
Source: Shutterstock.com
Stylised skeletal formula of levofloxacin, a third-generation fluoroquinolone. Atoms are shown as colour-coded circles: hydrogen (not shown), carbon (black), nitrogen (blue), oxygen (red).
Effectiveness of fluoroquinolones in the treatment of TB
WHO guidelines advise the use of fluoroquinolones in two main clinical contexts: (1) in the management of drug-sensitive TB as an alternative to first-line agents when these drugs are causing hepatotoxicity; (2) when treating MDR TB and XDR TB[25],
[26]
. Later-generation fluoroquinolones should be used in preference to earlier-generation ones in the treatment of MDR TB[14],
[16]
.
Levofloxacin is cheaper and generally more readily available than moxifloxacin[14]
. Fluoroquinolones should be incorporated into MDR TB regimens in the following order of preference: high-dose levofloxacin (500mg–1000mg daily), moxifloxacin and gatifloxacin[14]
. Information summarising the doses and drug levels of the fluoroquinolones is provided in Table 1[16],
[24],
[27],
[28]
.
| Drug | Recommended dose | Route | Target drug levels (mg/L) | Minimum inhibitory concentration of drug-susceptible Mycobacterium tuberculosis isolates (mg/L) | Minimum inhibitory concentration threshold for resistance (µg/ml) |
|---|---|---|---|---|---|
| Moxifloxacin | 400mg once daily | Oral or intravenous | 2.5–4 (peak) | 0.032–0.5 | > 8.0 |
| Levofloxacin | 10–15mg/kg once daily | Oral or Intravenous | 8–12 (peak) 0.5–2 (trough) | 0.125–0.5 | > 8.0 |
| Gatifloxacin | 400mg once daily | Oral | Unknown | 0.032–0.5 (extrapolated from values for moxifloxacin) | > 4.0 |
| Ofloxacin | 400mg twice daily | Oral or intravenous | Unknown | 0.25–1 | > 8.0 |
Historically, ciprofloxacin was commonly used. However, it has been shown to have poor in vivo activity[29]
and to select for M. tuberculosis strains that are ciprofloxacin-resistant[30]
. Furthermore, a review of clinical trials investigating the use of fluoroquinolones in pulmonary TB has shown that substituting older fluoroquinolones, such as ciprofloxacin, into treatment regimens results in a higher rate of relapse[31]
. Ciprofloxacin is, therefore, not recommended for use in WHO guidelines. Additionally, it is advised that ofloxacin be phased out of anti-MDR TB regimens[14]
.
Levofloxacin, the l-isomer of ofloxacin, has twice the activity of ofloxacin and thus better efficacy against ofloxacin-resistant strains of TB[32]
. The adverse effect profile of gatifloxacin, which includes severe dysglycaemia in an elderly population not being treated for TB[33]
, led to the parent company stopping its manufacture and resulted in a worldwide shortage of the drug. This means that moxifloxacin and levofloxacin are the fluoroquinolones of choice in therapy for TB; however, a preference between these latter two drugs is not clear based on current evidence.
Clinical trials evaluating the utility of fluoroquinolones in the treatment of TB
The potential use of fluoroquinolones as first-line agents to treat TB has been under investigation for several years[34]
. New-generation fluoroquinolones have been shown to be effective in treating M. tuberculosis in vitro
[35]
. Studies have demonstrated that fluoroquinolones exhibit intra-class cross-resistance[36]
, including between older drugs (e.g. ofloxacin) and later-generation fluoroquinolones[37]
.
There is an intrinsic appeal to shortening the duration of anti-TB therapy, as this would likely improve medication adherence, while also decreasing the risk of relapse and further spread of both drug-sensitive and drug-resistant TB[38]
. The possibility of using fluoroquinolones to reduce the duration of treatment for drug-sensitive TB was highlighted in a mouse study that examined outcomes using a regimen comprising two months of isoniazid, rifampicin and pyrazinamide, followed by four months of isoniazid and rifampicin (i.e. standard treatment) compared with a regimen where isoniazid was substituted with moxifloxacin. This demonstrated that murine TB was as likely to be cured by the four-month moxifloxacin-containing regimen as the standard six-month treatment combination[39]
.
Evidence for using fluoroquinolones for two months
TB in mice is not the same as that found in humans, and clinical trials focusing on the latter have shown mixed results. Studies have demonstrated that using gatifloxacin or moxifloxacin in place of ethambutol in the intensive phase of treatment for pulmonary TB results in a regimen that has an improved sterilising activity when compared with an intensive phase regimen that substitutes ethambutol with ofloxacin[40]
. Relapse following treatment was no different between two regimen given for nine months that used either ethambutol or ofloxacin for the entire duration of therapy[41]
. The use of moxifloxacin in place of ethambutol in a first-line TB treatment regimen has been shown to result in earlier sputum culture conversion, but has no effect on overall sputum culture conversion at two months[42]
.
In another Phase II study, moxifloxacin appeared to be better than ethambutol at achieving sputum conversion by the end of eight weeks of treatment, although there was no impact on overall treatment outcome[43]
. Substituting isoniazid with moxifloxacin has been shown to result in a statistically insignificant improvement in sputum culture conversion at eight weeks[44]
. The addition of levofloxacin to the treatment regimen does not produce a significant change in outcome in HIV-infected adults[45]
. A Cochrane review of the use of ofloxacin, levofloxacin, moxifloxacin and gatifloxacin in randomised controlled trials for treating drug-sensitive TB has shown that there is no improvement in outcomes such as relapse, treatment failure, sputum culture conversion at eight weeks or TB-related death, irrespective of whether the fluoroquinolones are added to the first-line regimen or substituted into it[21]
. Table 2 summarises the evidence for using fluoroquinolones for two months[40],
[42],
[43],
[44],
[45]
.
| Study | Summary |
|---|---|
| El-Sadr WM et al. (1998)[45] | The addition of levofloxacin to the treatment regimen did not improve sputum culture conversion at eight weeks |
| Burman WJ et al. (2006)[42] | Substituting ethambutol with moxifloxacin has no effect on sputum culture conversion at two months |
| Rustomjee R et al. (2008)[40] | Substituting ethambutol with gatifloxacin or moxifloxacin has better sterilising activity than substituting ethambutol with ofloxacin |
| Conde MB et al. (2009)[43] | Substituting ethambutol with moxifloxacin results in better sputum culture conversion at eight weeks but no impact on treatment outcome |
| Dorman SE et al. (2009)[44] | Substituting isoniazid with moxifloxacin does not produce a statistically significant improvement in sputum culture conversion at eight weeks |
Evidence for using fluoroquinolones to shorten the duration of anti-TB therapy
Several four-month treatment trials using fluoroquinolones have already been undertaken. Substituting ethambutol with gatifloxacin during the intensive phase of treatment, followed by continuing gatifloxacin during the continuation phase alongside rifampicin and isoniazid, has been shown to be no worse than the standard six-month WHO-recommended regimen. However, here the primary outcome measure was a composite of unfavourable outcomes (treatment failure, recurrence, or death or study dropout during treatment), and the authors attributed non-inferiority to the shorter regimen having a lower dropout despite a higher recurrence rate[46]
.
Another trial has shown that thrice-weekly regimens, using either gatifloxacin or moxifloxacin with isoniazid, rifampicin and pyrazinamide over four months, were inferior to the standard six-month treatment protocol, with the study being terminated early due to the number of relapses[47]
. Furthermore, a recent Phase III trial reported that while substituting ethambutol with moxifloxacin may result in a faster initial decrease in TB bacterial load, non-inferiority between the two different regimens was not demonstrable and the use of a four-month regimen containing moxifloxacin was less effective[48]
.
While the more rapid sputum conversion reported in several studies would suggest that the treatment duration could be shortened, this does not currently appear to be so. This may be due to dormant M. tuberculosis bacteria (requiring prolonged drug exposure) not being eliminated. To further investigate the utility of fluoroquinolones in shortening the duration of TB treatment, it would be useful to explore the clinical or laboratory markers that identify specific patients who may benefit from shorter treatment duration[49]
.
Studies have examined the potential of using drug regimens with less frequent drug dosing to achieve the same result. The utility of moxifloxacin in a novel treatment approach to TB has recently been highlighted: a six-month treatment regimen comprising two months of daily moxifloxacin, rifampicin, pyrazinamide and ethambutol followed by four months of once-weekly moxifloxacin and once-weekly rifapentine was found to be as effective as the standard WHO-mandated regimen[50]
. This may have important implications for improving treatment adherence and for treatment in areas of high isoniazid resistance. Table 3 summarises the studies using fluoroquinolones to shorten the duration of anti-TB therapy[46],
[47],
[48],
[50]
.
| Study | Summary |
|---|---|
| Jawahar MS et al. (2013)[47] | Adding gatifloxacin or moxifloxacin to rifampicin, isoniazid and pyrazinamide for four months was inferior to the standard six-month regimen |
| Merle CS et al. (2014)[46] | Substituting ethambutol with gatifloxacin was no worse than the standard six-month regimen |
| Gillespie SH et al. (2014)[48] | Substituting ethambutol with moxifloxacin for four months was less effective than the standard regimen |
| Jindani A et al. (2014)[50] | Using moxifloxacin in the intensive phase and having a continuation phase comprised of once-weekly moxifloxacin and once-weekly rifapentine is as effective as the standard regimen |
Evidence for using fluoroquinolones in MDR TB
The inclusion of fluoroquinolones in MDR TB treatment regimens has been shown to improve survival among MDR TB patients in Eastern Europe[51]
. Additionally, a meta-analysis indicated that newer generation fluoroquinolones are associated with both improved MDR treatment success and survival[52]
. There are no randomised studies available to directly compare the use of fluoroquinolones and second-line injectable agents. While issues relating to second-line injectable agents include their toxicity profile and difficulties in administering the drug, they have been used in the ‘9-month Bangladesh regimen’[17]
.
Table 4 summarises studies that have examined the use of fluoroquinolones in drug-resistant TB[51],
[52],
[53],
[54]
.
| Study | Summary |
|---|---|
| Ahuja SD et al. (2013) meta-analysis[52] | Later generation fluoroquinolones are associated with treatment success when used as part of multidrug regimens |
| Balabanova Y et al. (2016)[51] | Fluoroquinolones improve survival among multidrug-resistant TB (MDR TB) patients |
| Dheda K et al. (2010)[53] | Treatment with moxifloxacin is an independent predictor of survival in ofloxacin-resistant extensively drug-resistant TB (XDR TB) |
| Jacobson KR et al. (2010) meta-analysis[54] | Use of later generation fluoroquinolones in the treatment of XDR TB improves outcomes even though DST shows fluoroquinolone resistance |
Fluoroquinolones in CNS TB
While moxifloxacin has variable CNS penetration in animal studies[55],
[56]
, it has been shown to enter the CNS readily in humans and successfully treat TB meningitis[57]
; in particular when co-administered with higher-dose rifampicin[58]
. However, while levofloxacin exhibits greater human CNS penetration than either gatifloxacin or ciprofloxacin[59]
, a recent study has shown that an intensified TB meningitis treatment regimen comprising high-dose rifampicin and levofloxacin is not associated with improved survival compared with a standard treatment regimen[60]
.
Fluoroquinolones and hepatotoxicity in TB treatment
Despite hepatotoxicity being listed as an important adverse effect of fluoroquinolones (in particular with moxifloxacin), these drugs are often used when this has arisen secondary to other treatment with anti-TB drugs. Clinical studies suggest that there is a low risk of hepatotoxicity when moxifloxacin is used in TB treatment regimens[61]
. Levofloxacin and moxifloxacin have been shown to cause no additional hepatotoxicity in patients who have developed hepatitis secondary to first-line anti-TB drugs[62]
. Additionally, using moxifloxacin-based therapy in TB patients vulnerable to hepatic damage does not increase treatment-related hepatotoxicity[63]
.
Adverse effects and interactions of fluoroquinolones in the treatment of TB
As with any drug, it is important to balance the activity and effectiveness of fluoroquinolones against their adverse event profile and issues relating to tolerance[64]
. The incidence of adverse events associated with fluoroquinolone use is variable[65]
, although it should be noted that this is not specific to TB and that there is an element of specificity of adverse events associated with fluoroquinolones in TB treatment because of patients’ concomitant comorbidities and the other drugs that are being used.
There have been concerns regarding fluoroquinolone-induced arthropathy in children, although this is not supported by existing data[66]
. It has also been suggested that serum moxifloxacin concentration is reduced in HIV-positive children who are being treated for TB[67]
. It should be noted that the majority of studies have been in adults who have been carefully screened for comorbidities. Extra caution should be taken to monitor for adverse effects in the elderly population as they are at increased risk of potential fluoroquinolone-induced complications owing to comorbidities[68]
. Oral multivitamins and minerals may interfere with the absorption of fluoroquinolones, and it is important to ensure that these agents are taken several hours apart[69]
. The use of fluoroquinolones is advocated when treating MDR TB in pregnancy despite the limited data on the effect of their long-term use. However, they should be avoided if a woman is breastfeeding, owing to the ongoing concerns regarding arthropathy developing in the baby[16]
.
Fluoroquinolones are associated with a number of adverse effects[16]
. Moxifloxacin and gatifloxacin are more likely than other fluoroquinolones to cause electrocardiographic QT prolongation resulting in an increased risk of life-threatening arrhythmias. Special care is needed if fluoroquinolones are administered with the new anti-TB agents bedaquiline or delamanid, both of which are also known to prolong the QT interval[70]
. There is also a risk of QT prolongation when clofazimine and fluoroquinolones are co-administered[71]
. If the risk of the prolonged QT degenerating into torsades de pointes outweighs the benefits of continuing the fluoroquinolone, the drug should be stopped. Pre-existing long QT syndrome is a contraindication to commencing a patient on fluoroquinolone therapy. Owing to the particular risk of dysglycaemia with gatifloxacin[33]
, it is contra-indicated in patients with pre-existing diabetes. Dosing of levofloxacin and gatifloxacin should be reduced in the context of renal impairment.
Other adverse effects of fluoroquinolones include: seizures; arthralgia; tendonitis and tendon rupture (commoner in older patients, diabetics and those taking high-dose oral corticosteroids); pseudomembranous colitis; peripheral neuropathy; superficial fungal infections; psychotic symptoms; depression; and hepatitis. Even though fluoroquinolones are advocated for use in patients who have developed hepatitis secondary to first-line anti-TB drugs, fluoroquinolones themselves can result in a rise in hepatic transaminase levels[72]
by causing a hypersensitivity reaction characterised by eosinophilia[73]
. There are a few reported cases of clinically significant hepatotoxicity secondary to levofloxacin[74]
and gatifloxacin[75]
, although on the whole, fluoroquinolone-induced hepatotoxicity is still rare[76]
. Fluoroquinolone dose adjustment or cessation may be necessary depending on the severity of the adverse effects.
The interaction of fluoroquinolones with other drugs is another important consideration. The interaction with rifampicin is particularly noteworthy because rifampicin induces Phase II metabolic enzymes that metabolise moxifloxacin: this means that the co-administration of moxifloxacin with rifampicin causes a reduction in the plasma concentration of moxifloxacin[77]
, giving rise to a potential need to measure drug levels and increase the dose of moxifloxacin. Furthermore, antacids decrease the absorption of fluoroquinolones and should be avoided where possible[16]
.
Fluoroquinolone-resistant TB
Mutations in the gyrA and gyrB genes of M. tuberculosis confer resistance to fluoroquinolones. These mutations can be detected by molecular diagnostics, such as the GenoType® MTBDRsl (Hain Lifescience GmbH, Nehren, Germany) assay[78]
. There are concerns that the widespread use of fluoroquinolones to treat other infections[79]
may give rise to increasing fluoroquinolone-resistant TB, rendering these drugs ineffective against TB[38]
. This is compounded by the antibiotics being freely available in many parts of the world, meaning that they can be used when not clinically indicated and with minimal antibiotic stewardship[80]
. TB patients are three times more likely to have fluoroquinolone-resistant TB if they have previously been exposed to fluoroquinolones when compared with those who are fluoroquinolone-naïve[81]
. Of particular note is the empirical use of fluoroquinolones to treat lower respiratory tract infections, which may result in a delay in both the diagnosis and treatment of TB[82],
[83]
. The value of fluoroquinolones is highlighted by the empirical evidence that the use of these drugs in XDR TB treatment is associated with improved outcomes, even if DST appears to demonstrate fluoroquinolone resistance[84]
. However, if this is shown to have been acquired during treatment for MDR TB, the benefit is likely to be less apparent and this would lend itself to modification of the treatment regimen[16]
.
Future priorities
The 2016 document WHO treatment guidelines for drug-resistant tuberculosis – 2016 update has declared that fluoroquinolones are the most important component of an anti-MDR TB regimen, that their benefits outweigh their potential risks and that they should always be included in treatment regimens for MDR TB unless there is an absolute contraindication[14]
. Nevertheless, there remain a number of areas of research that need to be addressed.
One is their role (including the utility and adverse effects) in preventive treatment for contacts of patients with MDR TB[85]
. While there are data to suggest that levofloxacin may be of benefit in the treatment of latent TB[54]
, current research is limited. A recent cost-effectiveness analysis showed that fluoroquinolone preventive therapy for MDR TB contacts could result in a reduction in mortality and MDR TB incidence, as well as significant cost savings on drugs and delivery of healthcare services[86]
.
Research is currently under way to develop new fluoroquinolones with improved antimycobacterial activity[87]
. Other research priorities should include further profiling of the outcomes and adverse effects of using fluoroquinolones in new drug combinations to shorten the duration of TB treatment, which should facilitate improved treatment adherence and decrease the risk of relapse and further drug-resistant TB arising. There are already promising data in this area: a Phase IIb trial has shown that a combination of moxifloxacin, pretomanid (PA-824) and pyrazinamide is safe and effective in the treatment of both drug-susceptible and MDR TB[88]
.
Additional research is also needed on the use of fluoroquinolones to treat TB in adult patients with liver disease and other comorbidities, as well as in the paediatric population. Ultimately, new-generation fluoroquinolones should be used within effective anti-TB regimens that are short in duration, easy to take and have minimal adverse effects in all age groups[89]
.
Furthermore, increasing fluoroquinolone resistance continues to pose a threat to future TB treatment options. WHO recommends the use of the GenoType® MTBDRsl line probe assay as an initial test to detect fluoroquinolone resistance in patients with MDR TB, with there being a high degree of correlation between the fluoroquinolone resistance mutations detected by the GenoType® MTBDRsl line probe assay and the phenotypic resistance to ofloxacin and levofloxacin[90]
. This in turn will help to direct appropriate changes to any prescribed regimen.
Nevertheless, to continue to capitalise on the utility of fluoroquinolones in TB treatment, it will be necessary to comprehensively address the issue of fluoroquinolone resistance at its source and prevent further drug resistance from arising in the first place. To this end, the principles of antibiotic stewardship will need to be implemented on a global scale, supported by sustained funding for TB healthcare services and advocated by robust political leadership[91]
.
Financial and conflicts of interest disclosure:
Timothy D McHugh and Marc Lipman have been involved in clinical studies using fluoroquinolones to treat tuberculosis. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] World Health Organization. WHO Global Tuberculosis Report 2016. WHO, 2016. Available at: http://www.who.int/tb/publications/global_report/en/ (accessed May 2017)
[2] Mack U, Migliori GB, Sester M et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956–973. doi: 10.1183/09031936.00120908
[3] Lawn SD & Zumla AI. Tuberculosis. Lancet 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3
[4] World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision (updated December 2014). WHO, 2014. Available at: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf (accessed May 2017)
[5] Farmer PE, Furin JJ & Shin SS. Managing multidrug-resistant tuberculosis. J Respir Dis 2000;21(1):53–56.
[6] Kumar K & Abubakar I. Clinical implications of the global multidrug-resistant tuberculosis epidemic. Clin Med (Lond). 2016 Dec;16(6):565–570. doi: 10.7861/clinmedicine.16-6-565
[7] World Health Organization. Treatment of tuberculosis: guidelines. 4th edn. Document WHO/HTM/TB/2009.420. WHO, 2010. Available at: http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf (accessed May 2017)
[8] American Thoracic Society, CDC, Infectious Diseases Society of America. Treatment of tuberculosis. Morbidity and Mortality Weekly Report: Recommendations and Reports, 2003, 52(RR-11):1–77. PMID: 12836625
[9] Lienhardt C, Cook SV, Burgos M et al. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. JAMA. 2011 Apr 13;305(14):1415–1423. doi: 10.1001/jama.2011.436
[10] Albanna AS, Smith BM, Cowan D et al. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J 2013;42(3):721–732. doi: 10.1183/09031936.00180612
[11] Gallardo CR, Rigau Comas D, Valderrama RodrÃguez A et al. Fixed-dose combinations of drugs versus single-drug formulations for treating pulmonary tuberculosis. Cochrane Database Syst Rev. 2016;(5):1–142. doi: 10.1002/14651858.cd009913.pub2
[12] Zumla A, Chakaya J, Centis R et al. Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 2015;3(3):220–234. doi: 10.1016/S2213-2600(15)00063-6
[13] TB CARE I. International Standards for Tuberculosis Care, Edition 3. TB CARE I, The Hague, 2014. Available at: http://www.who.int/tb/publications/ISTC_3rdEd.pdf (accessed May 2017)
[14] World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis – 2016 update. WHO, 2016. Available at: http://apps.who.int/iris/bitstream/10665/250125/1/9789241549639-eng.pdf (accessed May 2017)
[15] National Institute for Health and Care Excellence (NICE) guideline [NG33] Tuberculosis. Available at: https://www.nice.org.uk/guidance/ng33/evidence/full-guideline-80851860868 (accessed May 2017).
[16] World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO, 2014. Available at: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf (accessed May 2017)
[17] Aung KJ, Van Deun A, Declercq E et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18(10):1180–1187. doi: 10.5588/ijtld.14.0100
[18] World Health Organization. The shorter MDR-TB regimen. WHO, 2016. Available at: http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf (accessed May 2017)
[19] Laurence YV, Griffiths UK, Vassall A. Costs to health services and the patient of treating tuberculosis: a systematic literature review. Pharmacoeconomics. 2015;33(9):939–955. doi: 10.1007/s40273-015-0279-6
[20] Lesher GY, Froelich EJ, Gruett MD et al. 1,8-Naphthyridine derivatives. A new class of chemotherapeutic agents. J Med Pharm Chem 1962;91:1063–1065. doi: 10.1021/jm01240a021
[21] Ziganshina LE, Titarenko AF & Davies GR. Fluoroquinolones for treating tuberculosis (presumed drug-sensitive). Cochrane Database Syst Rev 2013 Jun 6;(6):CD004795. doi: 10.1002/14651858.cd004795.pub4
[22] Mdluli K & Ma Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect Disord Drug Targets. 2007 Jun;7(2):159–168. doi: 10.2174/187152607781001763#
[23] Berning SE. The role of fluoroquinolones in tuberculosis today. Drugs 2001;61(1):9–18. doi: 10.2165/00003495-200161010-00002
[24] Angeby KA, Jureen P, Giske CG et al. Wildtype MIC distributions of four fluoroquinolones active against Mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamic data. J Antimicrob Chemother 2010;65:946–952. doi: 10.1093/jac/dkq091
[25] Caminero JA, Sotgiu G, Zumla A et al. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0
[26] Mitnick CD, Shin SS, Seung KJ et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359(6):563–574. doi: 10.1056/NEJMoa0800106
[27] Potter JL, Capstick T, Ricketts WM et al. TB Drug Monographs. Available at: http://www.tbdrugmonographs.co.uk/ (accessed May 2017)
[28] Heifets L. “Role of Phenotypic Methods for Drug Susceptibility Testing of M. Tuberculosis Isolates in the Era of MDR and XDR Epidemics.” Tuberculosis: Laboratory Diagnosis and Treatment Strategies. Ed. T. D. McHugh. 1st ed. Croydon, UK: CABI, 2013. 95–107. Print. doi: 10.1079/9781845938079.0095
[29] Shandil RK, Jayaram R, Kaur P et al. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 2007;51(2):576–582. doi: 10.1128/AAC.00414-06
[30] Gumbo T, Louie A, Deziel MR et al. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob Agents Chemother 2005;49:3178–3181. doi: 10.1128/AAC.49.8.3178-3181.2005
[31] Moadebi S, Harder CK, Fitzgerald MJ et al. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs 2007;67(14):2077–2099. doi: 10.2165/00003495-200767140-00007
[32] Yew WW, Chan CK, Leung CC et al. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest 2003;124(4):1476–1481. doi: 10.1378/chest.124.4.1476
[33] Park-Wyllie LY, Juurlink DN, Kopp A et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354(13):1352–1361. doi: 10.1056/NEJMoa055191
[34] Narayanan P. Shortening short course chemotherapy: a randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian J Tuberc 2002;49:27–38.
[35] Berlin OG, Young LS & Bruckner DA. In-vitro activity of six fluorinated quinolones against Mycobacterium tuberculosis. J Antimicrob Chemother 1987;19(5):611–615. doi: 10.1093/jac/19.5.611
[36] Von Groll A, Martin A, Jureen P et al. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob Agents Chemother 2009;53(10):4498–4500. doi: 10.1128/AAC.00287-09
[37] Devasia RA, Blackman A, May C et al. Fluoroquinolone resistance in Mycobacterium tuberculosis: an assessment of MGIT 960, MODS and nitrate reductase assay and fluoroquinolone cross-resistance. J Antimicrob Chemother 2009;63(6):1173–1178. doi: 10.1093/jac/dkp096
[38] Takiff H & Guerrero E. Current prospects for the fluoroquinolones as first-line tuberculosis therapy. Antimicrob Agents Chemother 2011;55(12):5421–5429. doi: 10.1128/AAC.00695-11
[39] Nuermberger EL, Yoshimatsu T, Tyagi S et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med 2004;170(10):1131–1134. doi: 10.1164/rccm.200407-885OC
[40] Rustomjee R, Lienhardt C, Kanyok T et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008;12(2):128–138. PMID: 18230244
[41] Kohno S, Koga H, Kaku M et al. Prospective comparative study of ofloxacin or ethambutol for the treatment of pulmonary tuberculosis. Chest 1992;102(6):1815–1818. doi: 10.1378/chest.102.6.1815
[42] Burman WJ, Goldberg S, Johnson JL et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006;174(3):331–338. doi: 10.1164/rccm.200603-360OC
[43] Conde MB, Efron A, Loredo C et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 2009;373(9670):1183–1189. doi: 10.1016/S0140-6736(09)60333-0
[44] Dorman SE, Johnson JL, Goldberg S et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med 2009;180(3):273–280. doi: 10.1164/rccm.200901-0078OC
[45] el-Sadr WM, Perlman DC, Matts JP et al. Evaluation of an intensive intermittent-induction regimen and duration of short-course treatment for human immunodeficiency virus-related pulmonary tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) and the AIDS Clinical Trials Group (ACTG). Clin Infect Dis 1998;26(5):1148–1158. doi: 10.1086/520275
[46] Merle CS, Fielding K, Sow OB et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371(17):1588–1598. doi: 10.1056/NEJMoa1315817
[47] Jawahar MS, Banurekha VV, Paramasivan CN et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS One 2013;8(7):e67030. doi: 10.1371/journal.pone.0067030
[48] Gillespie SH, Crook AM, McHugh TD et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371(17):1577–1587. doi: 10.1056/NEJMoa1407426
[49] Nimmo C, Lipman M, Phillips PP et al. Shortening treatment of tuberculosis: lessons from fluoroquinolone trials. Lancet Infect Dis 2015;15(2):141–143. doi: 10.1016/S1473-3099(14)70885-0
[50] Jindani A, Harrison TS, Nunn AJ et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014;371(17):1599–1608. doi: 10.1056/NEJMoa1314210
[51] Balabanova Y, Ignatyeva O, Fiebig L et al. Survival of patients with multidrug-resistant TB in Eastern Europe: what makes a difference? Thorax 2016;71(9):854–861. doi: 10.1136/thoraxjnl-2015-207638
[52] Ahuja SD, Ashkin D, Avendano M et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012;9(8):e1001300. doi: 10.1371/journal.pmed.1001300
[53] Dheda K, Shean K, Zumla A et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010 May 22;375(9728):1798–1807. doi: 10.1016/S0140-6736(10)60492-8
[54] Jacobson KR, Tierney DB, Jeon CY et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010;51(1):6–14. doi: 10.1086/653115
[55] Ostergaard C, Sorensen TK, Knudsen JD et al. Evaluation of moxifloxacin, a new 8-methoxyquinolone, for treatment of meningitis caused by a penicillin-resistant pneumococcus in rabbits. Antimicrob Agents Chemother 1998;42(7):1706–1712. PMID: 9661008
[56] Rodriguez-Cerrato V, McCoig CC, Michelow IC et al. Pharmacodynamics and bactericidal activity of moxifloxacin in experimental Escherichia coli meningitis. Antimicrob Agents Chemother 2001;45(11):3092–3097. doi: 10.1128/AAC.45.11.3092-3097.2001
[57] Alffenaar JW, van Altena R, Bökkerink HJ et al. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin Infect Dis 2009;49(7):1080–1082. doi: 10.1086/605576
[58] Ruslami R, Ganiem AR, Dian S et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5
[59] Thwaites GE, Bhavnani SM, Chau TT et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011;55(7):3244–3253. doi: 10.1128/AAC.00064-11
[60] Heemskerk AD, Bang ND, Mai NT et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016;374(2):124–134. doi: 10.1056/NEJMoa1507062
[61] Gillespie SH. The role of moxifloxacin in tuberculosis therapy. Eur Respir Rev 2016;25(139):19–28. doi: 10.1183/16000617.0085-2015
[62] Ho CC, Chen YC, Hu FC et al. Safety of fluoroquinolone use in patients with hepatotoxicity induced by anti-tuberculosis regimens. Clin Infect Dis 2009;48(11):1526–1533. doi: 10.1086/598929
[63] Roberts CH, Smith C, Breen R et al. Hepatotoxicity in the treatment of tuberculosis using moxifloxacin-containing regimens. Int J Tuberc Lung Dis 2011;15(9):1275–1276. doi: 10.5588/ijtld.11.0352
[64] Bryskier A & Lowther J. Fluoroquinolones and tuberculosis. Expert Opin Investig Drugs 2002;11(2):233–258. doi: 10.1517/13543784.11.2.233
[65] Mandell L & Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis 2002;13(1):54–61. doi: 10.1155/2002/864789
[66] Thee S, Garcia-Prats AJ, Donald PR et al. Fluoroquinolones for the treatment of tuberculosis in children. Tuberculosis (Edinb) 2015;95(3):229–245. doi: 10.1016/j.tube.2015.02.037
[67] Thee S, Garcia-Prats AJ, Draper HR et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis 2015;60(4):549–556. doi: 10.1093/cid/ciu868
[68] Stahlmann R & Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging 2010;27(3):193–209. doi: 10.2165/11531490-000000000-00000
[69] Sinclair D, Abba K, Grobler L et al. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2011;11:CD006086. doi: 10.1002/14651858.cd006086.pub3
[70] Gualano G, Capone S, Matteelli A et al. New antituberculosis drugs: from clinical trial to programmatic use. Infect Dis Rep 2016;8(2):6569. doi: 10.4081/idr.2016.6569
[71] Dannemann BR, Bakare N, De Marez T et al. Corrected QT Interval (QTcF) Prolongation in a Phase 2 Open-label Trial of TMC207 Plus Background Regimen (BR) as Treatment for MDR-TB: Effect of Co-administration with Clofazimine (CFZ). 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy: Abstract A1259.
[72] Bertino J Jr & Fish D. The safety profile of the fluoroquinolones. Clin Ther 2000;22(7):798–817; discussion 797. doi: 10.1016/S0149-2918(00)80053-3
[73] Orman ES, Conjeevaram HS, Vuppalanchi R et al. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol 2011;9(6):517–523.e3. doi: 10.1016/j.cgh.2011.02.019
[74] Figueira-Coelho J, Pereira O, Picado B et al. Acute hepatitis associated with the use of levofloxacin. Clin Ther 2010;32(10):1733–1777. doi: 10.1016/j.clinthera.2010.09.004
[75] Coleman CI, Spencer JV, Chung JO et al. Possible gatifloxacin-induced fulminant hepatic failure. Ann Pharmacother 2002;36(7-8):1162–1167. doi: 10.1345/aph.1A414
[76] Andrade RJ & Tulkens PM. Hepatic safety of antibiotics used in primary care. J Antimicrob Chemother 2011;66(7):1431–1446. doi: 10.1093/jac/dkr159
[77] Nijland HM, Ruslami R, Suroto AJ et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007;45:1001–1007. doi: 10.1086/521894
[78] Avalos E, Catanzaro D, Catanzaro A et al. Frequency and geographic distribution of gyrA and gyrB mutations associated with fluoroquinolone resistance in clinical Mycobacterium tuberculosis isolates: a systematic review. PLoS One 2015;10(3):e0120470. doi: 10.1371/journal.pone.0120470
[79] Ginsburg AS, Hooper N, Parrish N et al. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis 2003;37(11):1448–1452. doi: 10.1086/379328
[80] Schluger NW. Fluoroquinolones in the treatment of tuberculosis: which is best? Am J Respir Crit Care Med 2013;188(7):768–769. doi: 10.1164/rccm.201308-1446ED
[81] Migliori GB, Langendam MW, D’Ambrosio L et al. Protecting the tuberculosis drug pipeline: stating the case for the rational use of fluoroquinolones. Eur Respir J 2012;40(4):814–822. doi: 10.1183/09031936.00036812
[82] Chen TC, Lu PL, Lin CY et al. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2011;15(3):e211–6. doi: 10.1016/j.ijid.2010.11.008
[83] Craig SE, Bettinson H, Sabin CA et al. Think TB! Is the diagnosis of pulmonary tuberculosis delayed by the use of antibiotics? Int J Tuberc Lung Dis 2009;13(2):208–213. PMID:19146749
[84] World Health Organization. Guidelines on the management of latent tuberculosis infection. WHO, 2015. Available at: http://www.who.int/tb/publications/latent-tuberculosis-infection/en/ (accessed May 2017)
[85] Cremades R, RodrÃguez JC, GarcÃa-Pachón E et al. Comparison of the bactericidal activity of various fluoroquinolones against Mycobacterium tuberculosis in an in vitro experimental model. J Antimicrob Chemother 2011;66(10):2281–2283. doi: 10.1093/jac/dkr281
[86] Fox GJ, Oxlade O, Menzies D. Fluoroquinolone therapy for the prevention of multidrug-resistant tuberculosis in contacts. a cost-effectiveness analysis. Am J Respir Crit Care Med 2015;192(2):229–237. doi: 10.1164/rccm.201501-0069OC
[87] Guerrini V, De Rosa M, Pasquini S et al. New fluoroquinolones active against fluoroquinolones-resistant Mycobacterium tuberculosis strains. Tuberculosis (Edinb) 2013;93(4):405–411. doi: 10.1016/j.tube.2013.02.017
[88] Dawson R, Diacon AH, Everitt D et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015;385(9979):1738–1747. doi: 10.1016/S0140-6736(14)62002-X
[89] Rieder HL. Fourth-generation fluoroquinolones in tuberculosis. Lancet 2009;373(9670):1148–1149. doi: 10.1016/S0140-6736(09)60559-6
[90] World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO, 2010. Available at: http://apps.who.int/iris/bitstream/10665/44286/1/9789241599191_eng.pdf (accessed May 2017)
[91] Abubakar I, Zignol M, Falzon D et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis 2013;13(6):529–539. doi: 10.1016/S1473-3099(13)70030-6
You might also be interested in…
Norovirus and strategies for infection control

Case-based learning: insect bites and stings
