Custom Medical Stock Photo / Science Photo Library
Summary
Glaucoma is the second most common cause of blindness both in Europe and worldwide, and the most common cause of irreversible blindness in the world. The pathology of glaucoma is poorly understood, although the level of intraocular pressure (IOP) appears to be related to retinal ganglion cell death.
Primary open angle glaucoma is the most common form of glaucoma but is unusual in patients under 50 years of age. Disease progression depends on many factors, including age at time of diagnosis, difference between visual function at time of diagnosis and age-matched controls, and rate of progression (usually established in the first two years).
Closed angle glaucoma accounts for 50% of glaucoma blindness worldwide and is the most visually destructive form of glaucoma. It is caused by an occluded drainage angle, and glaucomatous damage will occur if the resulting rise in IOP is not dealt with promptly. Symptoms include pain, redness, blurring of vision or seeing halos, nausea and vomiting. However, some patients are asymptomatic. It is a medical emergency that requires urgent treatment.
Glaucoma is a group of optic neuropathies characterised by progressive degeneration of retinal ganglion cells. Retinal ganglion cells have their cell bodies in the inner retina and axons in the optic nerve. This degeneration results in cupping of the optic disc and visual loss
[1]
.
It is often associated with raised intraocular pressure (IOP) but this does not occur in up to 25% of cases of open angle glaucoma
[1]
.
Glaucoma can be classified into two broad types: open angle glaucoma (OAG), which accounts for 80% of cases, and closed angle glaucoma (CAG)[1]
. Both OAG and CAG can be primary disease with no identified cause. Secondary glaucoma has many causes, including medicines (e.g. corticosteroids), inflammation and tumours[2]
.
Glaucoma is the second most common cause of blindness both in Europe and worldwide, and the most common cause of irreversible blindness in the world. The reported percentage of patients affected depends on how glaucoma is defined; 70 million people worldwide are believed to be affected, with around 10% being bilaterally blinded. In the UK, it is estimated that 2% of the population aged over 40 years are affected, rising to almost 10% in patients aged over 75 years[1],[2],[3]
.
Age and ethnicity are strong risk factors for OAG. It has a prevalence of 3.4% in patients of European descent aged 74 years, increasing to 9.4% for those aged over 75 years, compared with 5.7% in patients of African descent aged 74 years, rising to 23.2% in those aged over 75 years[4]
. Other risk factors for OAG include increased cup-disc ratio (CDR), CDR asymmetry, disc haemorrhages, a family history of glaucoma, type 2 diabetes, myopia, thinner cornea, low ocular perfusion pressure (difference between blood pressure and IOP) and raised IOP[2],[4]
.
Population studies estimate that only 10–50% individuals with glaucoma are aware they have this condition, which means a large number of patients are not being appropriately treated and may suffer some vision loss[1]
.
Glaucoma can be hereditary, and patients aged over 40 years with first-degree relatives who have glaucoma are entitled to a free eye test in the UK.
Pathophysiology
The pathology of glaucoma is poorly understood, although the level of IOP appears to be related to retinal ganglion cell death.
The nerve fibres of the optic disc exit the eye posteriorly through a hole in the sclera that is occupied by a mesh-like structure called the lamina cribrosa, which is thought to maintain the pressure gradient between the inside of the eye and the surrounding tissues. The lamina cribrosa is more sensitive to pressure changes, which causes it to be displaced in high IOP, resulting in pinching of the optic nerve and blood vessels and possibly causing nerve damage. High IOP can also cause mitochondrial dysfunction in retinal ganglion cells in which energy demands are not met, leading to metabolic stress and nerve degeneration[1]
.
IOP is determined by the balance between the production of aqueous humour and its drainage via the trabecular meshwork and uveoscleral outflow pathway. In OAG, there is a resistance to aqueous outflow via the trabecular meshwork, whereas in CAG the pathway to the drainage channel is obstructed, usually by the iris (see ‘Closed angle glaucoma’).
Aqueous humour is produced by the ciliary body and at an average rate of 2.5 microlitres per minute. It is secreted into the posterior chamber of the eye and passes through the pupil into the anterior chamber, and then to the trabecular meshwork in the anterior angle.
The trabecular meshwork is composed of collagen and elastic tissue covered by trabecular cells that form a filter of decreasing pore size as the canal of Schlemm is approached[5]
. Any condition that causes blockage of the trabecular meshwork (e.g. debris following cataract surgery) can cause secondary glaucoma[2]
.
The uveoscleral outflow pathway refers to drainage of aqueous humour from the anterior chamber to the anterior angle other than through the trabecular meshwork. Unlike the trabecular meshwork, the uveoscleral pathway is not a distinctive pathway with defined structures and channels, but a route whereby aqueous humour seeps around and between tissues. The uveoscleral outflow is thought to be driven by pressure gradients by movement of the ciliary muscle[6]
.
The mean IOP in adults has been estimated to be between 15–16mmHg, with a standard deviation of nearly 3mmHg. Traditionally, the upper end of normal IOP has been defined as 21mmHg. IOP is significantly higher during the night and reaches its peak around 5am, then decreases during the day to produce a trough level at 9.30pm[2],[7]
.
Ocular hypertension is an elevated IOP above 21mmHg without disc or field changes, and is often a risk factor for glaucoma. The Ocular Hypertension Treatment Study (OHTS), which looked at patients with ocular hypertension, aimed to reduce IOP to less than 24mmHg or produce a decrease of IOP of at least 20% from baseline. After five years, 4.4% in the treatment group had developed glaucoma compared with 9% in the placebo group (50% relative risk reduction, hazard ratio 0.4, 95% confidence interval 0.27–0.59, P<0.001). After five years, the placebo group also started treatment to reduce IOP, and after 13 years, 22% of patients in the original placebo group had developed glaucoma compared with 16% in the original treatment group[8],[9]
.
Glaucomatous optic nerve damage can occur in patients with IOP in the normal range. These patients may have an abnormally low cerebrospinal fluid pressure in the optic nerve subarachnoid space, resulting in a large pressure gradient across the lamina cribrosa[1]
. Conversely, there are a significant number of patients with an IOP above 21mmHg (ocular hypertension) who do not develop glaucoma despite 20 years of follow up[2]
. The National Institute for Health and Care Excellence (NICE) recommends that all patients with an IOP above 32mmHg are treated regardless of symptoms. Patients with a central cornea thickness (CCT) between 555–590μm should receive treatment if their IOP is more than 22mmHg, and patients with a CCT below 555μm are treated if their IOP is more than 21mmHg.
Patients with type 2 diabetes are at increased risk of glaucoma as microvascular changes associated with diabetes are thought to lead to impaired microcirculation, which contributes to greater susceptibility of the optic nerve to damage. Patients with myopia are thought to have weaker scleral support at the optic nerve, which can also lead to nerve damage[4]
.
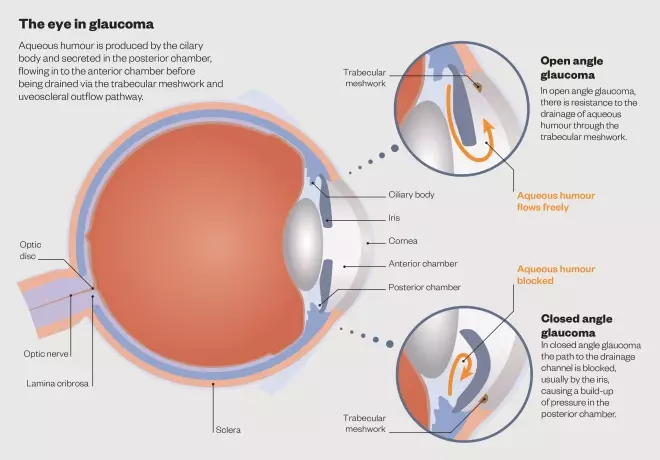
The eye in glaucoma
Aqueous humour is produced by the cilary body and secreted in the posterior chamber, flowing in to the anterior chamber before being drained via the trabecular meshwork and uveoscleral outflow pathway.
Primary open angle glaucoma
The most common form of glaucoma is primary OAG but it is unusual in patients aged under 50 years. It is a chronic progressive optic neuropathy, with a characteristic appearance of the optic nerve head and retinal nerve fibre layer in the absence of any other ocular disease or congenital abnormalities.
The relationship between structural and functional loss is weak, especially in early glaucoma. In some patients structural changes are seen but no functional loss, and vice versa. However, in advanced glaucoma there is increased correlation.
Disease progression depends on many factors, including age at time of diagnosis, difference between visual function at time of diagnosis and age-matched controls, and rate of progression (usually established in the first two years).
Risk factors for OAG include having a first-degree relative with OAG, high myopia, and low CCT (one reason is that this may increase the risk of missed diagnosis, resulting in a delay to treatment). Other factors that lead to an increased risk are less clear cut and less certain, such as ocular perfusion pressure, diabetes and systemic hypertension[2]
.
Primary congenital glaucoma is a rare form of glaucoma that occurs in children. It usually presents from birth up to two years of age, although it can be diagnosed later. It is caused by incomplete development of the trabecular meshwork and has a strong genetic component. If diagnosis is delayed, extremely extended globes can occur. Treatment is surgical[2]
.
Secondary open angle glaucomas
Exfoliative (pseudoexfoliative) glaucoma is caused by abnormal fibrillo-granular proteins being produced in the ocular and extraocular tissues. This material, together with pigment granules from the iris, accumulates on the anterior lens capsule and the trabecular meshwork and inhibits the drainage of the aqueous humour. Exfoliative glaucoma usually occurs in patients aged over 60 years, has a strong genetic component and produces higher IOP than primary OAG. There is often significant damage by the time the disease is diagnosed and it progresses rapidly, even in patients treated with IOP-lowering medicines[2]
.
Corticosteroid-induced glaucoma results from changes in the extracellular matrix (glycoproteins) caused by corticosteroids, which lead to decreased outflow facilities in certain predisposed patients. The risk of increased IOP depends on the strength of the corticosteroid, dose frequency, duration of therapy and route of administration. Risk factors for developing raised IOP with corticosteroids include a family history of glaucoma, diabetes, myopia, and young or old age. Elevated IOP usually develops two to six weeks after initiating corticosteroid therapy, but can occur at any time[2]
.
Obstruction of the trabecular meshwork can be caused by leaking lens material from a mature or hyper-mature cataract, traumatic lens injury or intraocular haemorrhaging. Uveitis can result in inflammatory cells, precipitates and secondary scarring, all of which can impede drainage of the aqueous humour.
Clinical features
Patients with OAG usually experience no symptoms until there is a significant sight loss. Currently, there is insufficient economic evidence on which to base recommendations regarding screening for OAG[4]
, although targeted screening of high risk groups (e.g. older patients of African descent) is currently being discussed.
The American Academy of Ophthalmology classifies OAG in three categories[4]
:
- Mild: optic nerve abnormalities consistent with glaucoma and a normal visual field test using standard automated perimetry (SAP);
- Moderate: optic nerve abnormalities with visual field abnormalities in one hemi-field;
- Severe: optic nerve abnormalities with visual field abnormalities in two hemi-fields.
Diagnosis
Early OAG can be challenging to diagnose as it is often symptomless until a large amount of neural damage has occurred, resulting in vision loss. A reduction in vision in one eye may be missed due to compensation by the other eye[2]
.
The main diagnostic tests are measurement of IOP, optic nerve assessment, and visual field measurement. NICE also recommends measurement of CCT (which can influence IOP results), peripheral anterior chamber configuration and depth assessment (which helps to establish if the patient has OAG or CAG)[10]
.
Examination of the optic nerve head and standard visual field testing are recommended to detect early disease. However, with the latter there needs to be a 30–50% retinal ganglion cell loss before changes can be detected[1],[2]
.
IOP is measured using a Goldmann applanation tonometer mounted on the slit lamp biomicroscope. The cornea is anaesthetised, and the tonometer head is used to flatten the cornea by a fixed amount; the force required to achieve this level of flattening is measured. The measurements can be influenced by corneal thickness; a thin cornea produces an artificially low measurement, whereas a thick cornea can produce an artificially high result[2]
,[10]
.
Optic head examination looks at the appearance of the optic nerve head and the retinal nerve fibre layer. Glaucoma causes narrowing of the neuroretinal rim or localised notching, which leads to cupping of the optic disc. Glaucoma can be missed in patients with small optic discs because there is ‘saucerisation’ rather than cupping, whereas patients with large discs have a narrow neuroretinal rim normally, which may be recorded as a false positive.
The European Glaucoma Society guidelines (EGS) recommend a baseline image (e.g. colour photographs or a scan of the optic nerve head) is taken to allow ophthalmologists to monitor disease progression[2]
.
Visual field testing is important in the diagnosis of glaucoma but it is more important in monitoring disease progression and the efficacy of treatment. The preferred method is static automated perimetry, as it is less subjective, the results are numerical and tools for computer-based interpretation are available. If abnormal results are obtained, it is important to rule out other diseases or conditions that could influence these results (e.g. tumours, macular degeneration, diabetes).
Ideally, all newly diagnosed glaucoma patients should have three standard automated perimetry tests per year during the first two years following diagnosis to identify eyes showing a fast rate of progression at an early stage. Rapidly progressing eyes need more aggressive treatment, a lower target IOP and more frequent monitoring[2]
.
Gonioscopy is used to inspect the anterior chamber angle of the eye. Patients with narrow angles are at increased risk of acute angle closure which, if not treated promptly, leads to damage and development of glaucoma. OAG and CAG are treated completely differently so it is important to differentiate the conditions promptly[2]
.
Optic disc haemorrhages are more common in glaucoma patients, but they are not unique and are often associated with disease progression[2]
.
Closed angle glaucoma
Accounting for 50% of all glaucoma blindness worldwide, closed angle glaucoma (CAG) is the most visually destructive form of glaucoma. It is caused by an occluded drainage angle and glaucomatous damage will occur if the resulting rise in intraocular pressure (IOP) is not dealt with promptly. Usually, the cause of the obstruction is the peripheral iris but it can be caused by the formation of synechiae (adhesions between the iris and the cornea or the lens), which can occur in uveitis.
The very high IOP in CAG may cause ischaemic damage to the iris, necrosis of the lens epithelium leading to opacities, and damage to the trabecular meshwork and optic nerve.
Primary CAG is more common in people aged over 40 years, and is more common in patients of Asian (prevalence 1.4%) and Greenland Inuit (5%) ethnicity than in Europeans (0.1%)[2]
. Around 75% of cases occur in women. CAG is more common in patients who have a shallow anterior chamber.
Causes
Primary CAG occurs in the absence of ocular disease; if it is associated with ocular disease it is known as secondary CAG[1],[2]
. Several factors can cause the iris to obstruct the anterior angle, the most common being pupillary block. This is where the flow of aqueous humour from the posterior chamber through the pupil to the anterior chamber becomes impeded, causing a build-up of pressure in the posterior chamber. This results in the iris becoming bowed and coming into contact with the trabecular meshwork, inhibiting drainage. In a minority of cases, this become a self-perpetuating cycle leading to very high IOP (i.e. 50–80mmHg). An obstruction can also be caused if the thickness of the lens increases in individuals with a shallow anterior chamber[1],[2]
.
Drugs that dilate the pupil because of a sympathomimetic or anticholinergic activity can also trigger acute angle closure in susceptible individuals. These include topical mydriatics, nebulised ipratropium bromide, tricyclic antidepressants, selective serotonin reuptake inhibitors and topiramate. Unless individuals have first-degree relatives who have had CAG, many susceptible patients are not aware they have this condition and cannot be easily identified as a group to avoid these drugs. Once these patients have had CAG and are treated with an iridotomy, mydriatic drugs will have little effect[2]
. Patients with open angle glaucoma will not be affected by these drugs either.
Diagnosis
Symptoms include pain, redness, blurring of vision or seeing halos. However, the sensitivity and specificity of these symptoms are poor, and some patients are asymptomatic[1],[2]
. Some patients may experience nausea and vomiting. This is a medical emergency and requires prompt assessment and treatment in accident and emergency[2]
.
Gonioscopy is the standard technique for examining the anterior chamber angle, identifying contact between the iris and trabecular network.
Treatment
The main aim of treatment is to lower the IOP and reduce inflammation so that laser iridotomy or surgical iridectomy (hole in iris) is possible[2]
.
Systemic osmotic diuretics (e.g. oral glycerol or intravenous mannitol) are used to withdraw fluid from the posterior chamber. Care needs to be taken in patients with kidney or heart disease as osmotic diuretics will initially increase blood volume.
Corticosteroids (e.g. topical prednisolone 0.5% every five minutes for three applications, then four to six times a day) are used to reduce inflammation.
Acetazolamide (orally or intravenously 10mg/kg), in addition to topical beta blockers and topical alpha2-adrenoceptor agonists, are used to reduce fluid production in the eye[2]
.
Pilocarpine 2% eye drops are used to constrict the pupil and open the anterior chamber angle. However, it is ineffective until a decrease in pressure has been achieved with other medicines.
Once one eye has been treated, it is recommended that a prophylactic iridotomy is performed on the other, unaffected eye. Some patients who are considered at high risk of developing primary CAG are offered a prophylactic iridotomy to both eyes[2]
.
Elaine Mann is an ophthalmic, ENT, maxillofacial and dental pharmacist at Leeds Teaching Hospitals NHS Trust.
References
[1] Weinreb RN. The pathophysiology and treatment of glaucoma. A review. JAMA 2014;311(18):1901–1911.
[2] European Glaucoma Society. Terminology and Guidelines for Glaucoma 4th Edition.
[3] The Royal College of Ophthalmologists. Glaucoma general description.
[4] American Academy of Ophthalmology. Preferred practice pattern. Primary open-angle glaucoma. 2010.
[5] Riordan Evra P & Cunningham E. Vaughan & Asbury’s General ophthalmology. 16th edition. New York: Lange 2004.
[6] Toris CB. Uveoscleral outflow. Glaucoma Today 2013:36–37.
[7] Bartlett JD & Jaanus SD. Clinical ocular pharmacology 5th edition. Chicester: Elsevier 2008.
[8] Kass MA, Heuer DK, Higginbotham EJ et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):701–713.
[9] Kass MA, Gordon MO, Gao F et al. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol 2010;128(3):276–287.
[10] National Institute for Health and Care Excellence. Glaucoma: Diagnosis and management of chronic open angle glaucoma and ocular hypertension. London: NICE 2009.