Science Photo Library
Haematopoietic stem cell transplant (HSCT) is a highly specialised treatment for the management of a wide range of blood cancers, and other immunological and metabolic disorders[1],[2]
. The process involves destroying unhealthy blood cells and replaciÂng them with stem cells removed from the blood or bone marrow[1],[2]
. There are two main types of transplant categorised by the origin of the stem cells:
- Autologous — the stem cells in autologous transplants come from the patient themself, meaning the patient is their own donor.
- Allogeneic — the stem cells in allogeneic transplants are from a different person, either a matched related or unrelated donor[3]
.
HSCT can be used to treat conditions affecting the blood cells, such as leukaemia and lymphoma[1],[2]
. The procedure has significant risks (e.g. graft versus host disease [GvHD], infections [see Monitoring and complications]) and is therefore an intensive and challenging experience for both patients and healthcare professionals[2]
. This article will focus on allogeneic transplant and its complications.
Most patients stay in hospital for at least four weeks after the transplant until initial count recovery (i.e. the rise of neutrophils and lymphocytes to normal range) and engraftment (i.e.when the body accepts the transplanted bone marrow or stem cells, and they begin to produce new blood cells and immune system cells)[4]
. However, there is the expectation that full immune reconstitution may take up to two years, with the potential for numerous hospital readmissions for post-transplant complications[4]
. Despite extensive developments and treatment options in recent years, HSCT remains a complex and unpredictable procedure, with little understanding of why some patients have complications while others do not[4]
. However, the procedure itself may be more complex owing to:
- Existing condition leading to patient being heavily pre-treated (e.g. treated with intensive chemotherapy for leukaemia and relapsing before coming to transplant, which is a last resort for these high-risk patients);
- Pharmacokinetic and pharmacodynamic differences compared with adult patients, because children are from different age groups and organs such as kidneys and liver may not be fully developed;
- Delivery method (e.g. intravenous [IV] delivery of blood products and total parenteral nutrition [TPN]) requiring specialist pharmaceutical advice. These patients are on at least seven to eight IV medicines, excluding TPN and blood products, and require input from the pharmacist for IV compatibility.
This article outlines the pharmacist’s role during HSCT in children and uses Great Ormond Street Hospital (GOSH) as an example to explore the patient journey and actions of the multidisciplinary team (MDT) — which includes doctors, nurses, dietician, microbiologist, pharmacist and play therapist — specifically highlighting those of the pharmacist. The GOSH HSCT service is accredited by the joint accreditation committee of the international European Society for Blood and Marrow Transplantation (EBMT) (JACIE) — this is a regulatory body similar to the Medicines and Healthcare products Regulatory Agency and all centres offering a stem cell transplant service must have this accreditation.
Prior to admission
The parents/carers and the child are counselled about HSCT at their initial consultation visit by the consultant. They then make a joint decision as to how they want to move forward. If the agreement is on transplant, then the child and their parents/carers are invited to visit the ward to meet key members of the team (e.g. doctors, nurses, pharmacists, dietician and play therapist). This is to help familiarise them with the ward facilities, introduce them to key personnel, and alleviate some of the anxiety that the child and parents/carers might have.
At this stage, parents/carers frequently request information and advice related to medicines and treatment options.
Medicines
A common concern is the drugs administered during transplant, including any potential short- and long-term side effects. Parents/carers are often concerned about the number of complex medicines required and have understandable anxiety about this. Pharmacists will take time to go through the medicines, provide information about them and guide parents/carers to appropriate information sites to help them understand more about the medicines to be used.
The allergy status of the child is confirmed at this stage, to allow for any adjustments to medicines prescribed.
Herbal interactions
Parents/carers may ask for information about the risks and complications of any potential drug interactions with herbal/homeopathic medicine that the child may be taking. It is recommended that any herbal/homeopathic medicine is investigated to ensure safe practice[5]
. In practice, any interactions found should be highlighted to the parent/carer and appropriate advice relating to discontinuation given. When parents/carers are aware of risks and complications, they are usually compliant with the information provided[6]
.
Administration
At this stage, it is essential to discuss with individual families whether their child will be able to take numerous oral medications, both in transplant, but also for several months after discharge. This may be difficult because of patient age and schooling. Children may have the option to have a nasogastric (NG) tube inserted on admission, and for those who may not tolerate this, the insertion of a percutaneous endoscopic gastrostomy (PEG) tube may be required (this is often discussed in the pre-transplant meeting with the HSCT consultant or clinical nurse specialist [CNS]). While NG and PEG tubes are primarily inserted to ensure adequate nutrition, they are also indispensable for medicines administration, and when talking to parents/carers, they often prefer to keep the NG/PEG until the medicine burden is reduced. Families should be counselled to be realistic about the capabilities and willingness of their children in taking oral medicines for extended periods. Children often feel overwhelmed by these expectations and the pharmacist has an important role in empowering them and their families to regain some control over their transplant experience, by discussing their options and respecting their preference for certain formulations (e.g. liquids, tablets, capsules) and administration times[7]
.
Excipients
Families may be concerned about medicines and their excipients, specifically about their compatibility with religious beliefs and medical conditions. For example:
- Halal/kosher restrictions;
- Patients with phenylketouria or G6PD deficiency;
- Those with prolonged QT interval;
- Those with renal or hepatic impairment.
At GOSH, in the case of religious restrictions, drug companies are contacted and an alternative formulation is sought. Where no alternatives are found, and parents/carers feel very strongly, advice from the hospital ethics committee is sought.
Establishing both the clinically relevant and family information beforehand enables effective and robust forward planning, ensuring that all medicines are rationalised, modified or substituted when the child’s individual protocol is discussed and finalised.
Transplant protocol
Prior to admission (usually one to two weeks before), the patient’s individualised HSCT protocol is discussed and checked at the weekly MDT protocol meeting. Patients’ protocols are individualised because there is variability in the:
- Donor (matched sibling donor, matched unrelated donor or haploidentical);
- Source of stem cells (marrow, peripheral blood or cord);
- Underlying disease.
The MDT protocol meeting comprises two or more consultants (one of whom would have seen the patient at the initial pre-admission visit), the CNS, the pharmacist and the clinical fellows who will have daily direct clinical contact with the family. The pharmacist’s responsibility at this meeting is to check the protocol for:
- The practicalities of chemotherapy and drug administration — advising nursing staff about the appropriate timings and compatibilities of IV drug infusions. Drug administrations in this setting are complex, and are often restricted by time and appropriate IV access, including central venous access;
- Timing issues — proactive forward planning is essential to pre-empt any issues that may occur and plans made to address them appropriately (e.g. a short expiry of prepared cytotoxic drugs may create the need for manufacturing chemotherapy at the weekend, which will require planning to avoid wastage or drug errors);
- Foreseeable drug interactions or dose modifications — these may be based on the child’s age/weight, renal and/or liver function or any other pre-existing conditions.
Any amendments discussed and agreed will need to be checked by both the consultant and pharmacist. At GOSH, a copy of the final protocol is given to the parents/carers so they can follow the administration of chemotherapy and the transplant. A copy is also available to the pharmacist for prescribing and preparation of the chemotherapy.
After the protocol is released (two to seven days before the child’s planned admission), it is reviewed again thoroughly from start to finish and it is confirmed that all medications (prophylactic and treatment [including all chemotherapies]) are prescribed — with specific start and finish dates (see Table 1)[8],[9],[10],[11],[12],[13],[14],[15]
. This practice at GOSH was developed through trial and error — there were previous incidents where medications were not prescribed and inadvertently omitted. As a result of medication errors, near misses and a risk analysis meeting, the MDT agreed to prescribe the whole protocol in one sitting, with all medicines being prescribed by one prescriber to minimise incidents. The protocol is prescribed in advance of the child’s admission to reduce delay in manufacturing chemotherapy.
| Table 1: Commonly used prophylactic medicines in haematopoietic stem cell transplant | |||||
|---|---|---|---|---|---|
| Medicines | Start date | Stop date | Dose | Rationale | |
| Oral antifungal itraconazole is first line at GOSH | Usually two weeks prior to admission | After CD4 >300u/L | Initial starting dose 5mg/kg once daily | If therapeutic level is achieved, this is continued. | |
| Ambisome | On D-1 | Discontinue once patient is eating and drinking post-transplant and oral antifungal within therapeutic range (itraconazole is first line at GOSH) | Initiated at 1mg/kg/day |
| |
| Oral ciprofloxacin | On admission | When neutrophil count is >1 x 109/L in older children. In younger children, this is continued until the Hickman line is removed or the child has been weaned off nappies (the end of the Hickman line may frequently get contaminated by falling into the nappies and children end up with an E.coli system infection) |
| ||
| IV aciclovir | On D-1 if on fludarabine; on D-5 if not on fludarabine | CD4 count >300/µL (until at least one year post-HSCT) | Dose 250mg/m2 IV or 300mg/m2 orally three times per day |
| |
| Oral co-trimoxazole | Low dose on admission; restarted around D+28 | Stop at D-1 When CD4 count >300µL | Low dose of 7.5mg/kg twice daily (Dose: surface area <0.5m2 —15mg/kg/dose; 0.5–0.75m2 — 240mg; 0.76–1.0m2 — 360mg; >1.0m2 — 480mg. All doses are given Monday to Tuesday twice daily) |
| |
| Oral calcium folinate | In conjunction with co-trimoxazole (i.e. D+28) | When co-trimoxazole is stopped | Dose given is 15mg/kg once weekly | Decrease the myelosupressive side effects of co-trimoxazole. | |
| IV ciclosporin | On D-3 | Variable depending on diagnosis and GvHD | Starting dose at 1.5mg/kg twice daily | Prophylaxis of GvHD. | |
| IV mycophenolate | On D0 | Variable depending on diagnosis and GvHD | 15mg/kg IV/oral three times per day (max 2g/day) | Prophylaxis against GvHD and graft rejection. | |
| IV vitamin K* | Weekly on Friday from admission | At discharge | 0.3mg/kg (max 10mg) weekly |
| |
| Oral phenoxymethylpenicillin | Around D+28 | Life-long | See BNFc |
| |
| IV vancomycin* | On D0 | When neutrophil count is >0.2 x 109/L | 400mg/m2 twice daily |
| |
| Immunoglobulin** | On admission for immune-deficient patients undergoing HSCT, or when required for non-immune-deficient patients | Stop upon immune reconstitution | 0.5mg/kg every three weeks | To protect from opportunistic infections if severely immunosuppressed (e.g. for chronic GvHD). | |
| Oral clonazepam | 24 hours before busulfan | 48 hours after busulfan | 12.5 μg/kg orally twice daily; max 1mg/day) | Protects against busulfan-related seizures. | |
| Oral ursodeoxycholic | On admission | D+28 | 10mg/kg twice daily (dose can be increased to 15mg/kg three times per day) |
| |
| Sources: Lancet Oncol [8] , Great Ormond Street Hospital[9] , Ann Intern Med [10] , MMWR Recomm Rep [11] , Blood [12] , EBMT Handbook [13] , Blood [14] , Br J Haematol [15] D-1: day before transplant; D0: day of transplant; D+28: day 28 of transplant GOSH: Great Ormond Street Hospital VOD: veno-occlusive disease PCP: pneumocystis carinii pneumonia GvHD: graft versus host disease TBI: total body irradiation IV: intavenous HSCT: haematopoietic stem cell transplant CMV: cytomegalovirus CD4: a type of white blood cell, called T-cells, that move throughout the body to find and destroy bacteria, viruses and other invading germs *This is GOSH policy — may not be routinely used in all HSCT units **Usage of IV immunoglobulin post-HSCT is under review owing to a national shortage (the medicines are started at different times during the treatment to minimise toxicity and are stopped when patient has sufficient count recovery and/or is not at high risk of infection or complications) | |||||
On admission
On admission, the child is re-weighed and any adjustments for chemotherapy drug doses can be made prior to manufacturing, if required[9]
. The availability of a specialist pharmacist to discuss any concerns is an invaluable source of support for families. It is good practice for the pharmacist to meet the child and parents/carers to develop a relationship of trust with them. The pharmacist may have briefly met the family prior to admission, so building on that initial meeting is essential as the child progresses through the treatment regimen — to revisit and reassure about any new issues or concerns, and to ensure that any problems identified are addressed promptly.
Once the protocol has been finalised and medicines prescribed accordingly, the next step is to monitor and manage any complications that may arise during transplant[16]
. It is good practice to pre-empt complications (e.g. nausea, vomiting, mucositis, infection) and proactively work towards managing symptoms and minimising side effects (e.g. rationalising anti-emetics, adding appropriate analgesia).
As the child progresses through the transplant trajectory, their needs will change. During the early stages of transplant, when the child is going through their conditioning chemotherapy in preparation for the new cells (from admission to the day of transplant), they will primarily be at risk of chemotherapy-induced side effects (e.g. nausea, vomiting, mucositis, infection)[16]
. The role of the specialist pharmacist at this point is to ensure that those side effects are being appropriately managed; for example, adequate analgesia for mucositis (e.g. oral morphine or patient-controlled analgesia) and adequate anti-emetic cover for managing chemotherapy-induced nausea (e.g. adding in cyclizine, hyoscine patch).
Monitoring and complications
From day of transplant (D0) until neutrophil count recovery (approximately D+12 to D+21, depending on cell source and conditioning regimen), the child will have profound neutropenia and lymphopaenia, and is therefore at high risk of developing bacterial, fungal and viral infections[2],[17]
.
Monitoring must be robust (see Figure 1), and timely treatment of opportunistic infections is essential during this phase, when the risk of life-threatening infections is high[17]
. During this period, the child may also experience other transplant complications, such as veno-occlusive disease (VOD), posterior reversible encephalopathy syndrome (PRES) and transplant-associated thrombotic microangiopathy (TA-TMA)[20]
, all of which require extensive and complex drug regimens and monitoring (see Table 2)[[11],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28]
.
As the neutrophil count starts to recover (usually D+20), the child may be at additional risk of engraftment syndrome or GvHD (Figure 1)[17]
.
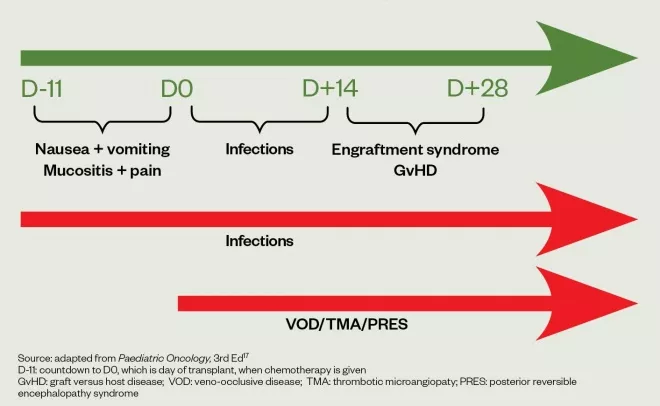
Figure 1: Guide to pre-empting and monitoring complications
Caring for a child undergoing an HSCT is challenging, and requires comprehensive and frequent monitoring. Table 2 summarises the monitoring required by the pharmacist during their daily ward visit, the rationale and any interventions that can be made.
| Table 2: Summary of monitoring for complications during haematopoietic stem cell transplant | |||
|---|---|---|---|
| Monitor | Frequency | Rationale | Intervention |
| Electrolytes (e.g. potassium, sodium, calcium, magnesium, phosphate) | Daily |
|
|
| Urea; creatinine | Daily |
|
|
| Liver function tests | Daily |
|
|
| Full blood count | Daily |
|
|
| Blood pressure; respiratory rate | Daily |
|
|
| Weight/girth | Daily |
|
|
| Fluid input/output | Daily |
|
|
| Mouth score | Daily |
|
|
| Viral counts in blood | Weekly, or twice weekly if previously positive |
|
|
| Therapeutic drug levels | As and when required |
|
|
| Skin rash | As and when required if having thiotepa, treosulfan or graft versus host disease |
|
|
| Blood in urine | Daily through cyclophosphamide conditioning; as and when required for BK viraemia |
|
|
| Lactate dehydrogenase enzyme | Twice weekly and daily if rising |
|
|
| Sources: Ann Intern Med [11] , Pinkerton et al . [17], Br J Haematol [18], Neurology [19], Transfus Apher Sci [20], EBMT Handbook [21], BNFc [22], BMJ [23], Nursing Times [24], Eur J Cancer[25], Br J Haematol [26], J Urol [27], BNFc [28] GCSF: granulocyte colony-stimulating factor GvHD: graft versus host disease IV: intravenous PCP: pneumocystis carinii pneumonia PRES: posterior reversible encephalopathy syndrome TPN: total parental nutrition | |||
Nausea and vomiting
Nausea and vomiting will be experienced by a majority of patients receiving chemotherapy and/or radiotherapy[2],[3],[29]
. The HSCT procedure has a high emetogenic risk factor owing to the high doses of combination chemotherapy and/or total body irradiation (TBI21). Anti-emetics are initiated 24 hours before conditioning therapy, because nausea and vomiting are easier to prevent than to treat[21],[30]
. The child’s previous history/experience of nausea and vomiting should be explored when meeting them on the day of admission. For example, the history of haematology patients who have previously had chemotherapy may provide insight into what would be an effective anti-emetic treatment.
The degree of nausea and vomiting will vary with each child, but may be worsened by anxiety and stress[31]
. When vomiting is intense, some of the other oral prophylactic medicines are reviewed and switched to IV therapy in the acute phase. Medicines that are only available as oral formulations may be withheld for a short period of time[9]
. For example, ursodeoxycholic acid for prophylaxis of VOD, and oral ciprofloxacin for gut decontamination, would be withheld and restarted once the child is able to tolerate oral medicines again.
At GOSH, fluid charts are checked daily for the number of emesis episodes. Discussions are held with the MDT and parents/ carers (patients may also be involved) to recommend/amend anti-emetics appropriately. IV or transdermal routes are almost always considered in the acute phase[21]
. If a patient is on multiple anti-emetic treatments, it is good practice to modify administration times, so the patient has good anti-emetic cover every 2–4 hours over a 24-hour period. It is important to monitor and treat any complications of nausea and vomiting, which may include dehydration, electrolyte disturbance and nutritional intake[21]
. Nausea and vomiting can be distressing and consideration should be given to alternative options, such as:
- Distraction therapy (i.e. enagaging the child with the play therapist, either painting or creating other form of artwork);
- Guided imagery (e.g. story/poem writing);
- Music therapy (i.e. enagaging children to partake in music playing with the therapist)[19],[32]
.
Mucositis
Mucositis is a painful inflammation and ulceration of the mucosal membrane that lines the gastrointestinal tract, conferring a potential risk of infection[33]
. This is caused by breakdown of epithelial cells by radiotherapy and/or chemotherapy[33]
. In addition, chemotherapy and radiotherapy can affect saliva production and cause mucosal damage[34]
. Symptoms may include difficulty swallowing, which includes the patient’s own saliva (it should be noted if the child has saliva dribbling down the side of their mouth); epigastric and lower abdominal pain; diarrhoea; and constipation[34]
. The degree of mucositis can change rapidly owing to the secondary effect of cytotoxic-induced myelosuppressive neutropenia; therefore, regular assessment (e.g. daily), evaluation and treatment is critical for effective care[34]
.
Mouthcare is usually performed by nurses on the ward[33]
. Parents/carers should be taught how to keep the oral mucosa clean and lips soft, moist and intact, as this can help prevent infection. In addition, they should be shown how to remove food debris and dental plaque without damaging the mucosa.
As pain assessment is subjective, it is important to use an oral mouth assessment guide (see Table 3)[25],[35]
. A total score of more than eight suggests that the child is at risk of complications and pain[25],[35]
. At GOSH, an analgesia (e.g. oral morphine) is prescribed when initially required and pain is monitored. If the doses are needed more frequently, then regular slow-release morphine sulphate is prescribed[9]
. Non-steroidal anti-inflammatory drugs (NSAIDs) or rectal administration of medicines are not used at GOSH, as there may be risk of bleeding and infection if the child is neutropenic. Paracetamol is not used as a first-line treatment in this case, as it may mask an increased temperature, and hence infection, as the child may be neutropenic or have lymphopenia[2]
. If the child has nausea or vomiting, bleeding gums or severe mucositis, the use of an IV opioid nurse controlled/patient-controlled analgesia syringe pump would be initiated[36]
. Pharmacists can recommend that sucking ice lollies or ice cubes may temporarily help numb the pain[37]
.
With painful bleeding gums, the child may not feel like eating or drinking and may start to lose weight. TPN may be considered if the child loses 10% of their body weight from admission[38]
.
| Table 3. Oral assessment guide for children and young people | ||||
|---|---|---|---|---|
| Category | Method of assessment | 1 | 2 | 3 |
| Swallow | Ask the child to swallow, or observe swallowing process. Ask the parent/carer if there are any notable changes | Normal Without difficulty | Difficulty swallowing | Unable to swallow at all. Observe drooling and dribbling of secretions |
| Lips and corner of mouth | Observe appearance of tissue | Normal Smooth, pink and moist | Dry, cracked or swollen | Ulcerated or bleeding |
| Tongue | Observe the appearance of the tongue using a pen-torch to illuminate | Normal Firm without fissures (cracking or splitting) or prominent papilla Pink and moist | Coated or loss of papillae with a shiny appearance, with or without redness and/or oral candida | Ulcerated, sloughing or cracked |
| Saliva | Observe consistency and quality of saliva | Normal Thin and watery | Excess amount of saliva, drooling | Thick, ropy or absent |
| Mucous membrane | Observe appearance of tissue using a pen-torch to illuminate the oral cavity | Normal Pink and moist | Reddened or coated without ulceration and/or oral candida | Ulceration and cloughing, with or without bleeding |
| Gingivae | Observe appearance of tissue using a pen-torch to illuminate the oral cavity | Normal Pink or coral with a dotted surface Gum margins tight and well defined | Oedamatous with or without redness, smooth | Spontaneous bleeding |
| Teeth (if no teeth, score 1) | Observe appearance of teeth using a pen-torch to illuminate the oral cavity | Normal Clean and no debris | Plague or debris in localised areas | Plaque or debris generalised along gum line |
| Voice | Talk and listen to the child. Ask the parent/carer if there are any notable changes | Normal tone and quality when talking or crying | Deeper or raspy | Difficult to talk, cry or not talking at all |
| Sources: Gibson et al.[25] ; Great Ormond Street Hospital[35] NB: If score is >8, introduce pain assessment instrument | ||||
Infections
Infections constitute a major cause of morbidity and mortality in all patients undergoing HSCT[39]
. The incidence and severity of infections strongly correlate with the use of immunosuppressants and timing of neutrophil recovery and immune reconstitution[17]
. Prophylaxis with effective antifungals (e.g. azoles for fungal infections), antivirals (e.g. aciclovir for varicella-zoster virus), and antibiotics (e.g. co-trimoxazole for pneumocystis carinii pneumonia [PCP]) decrease the incidence of some infections during HSCT[26]
. Key considerations when choosing prophylactic medications include its:
- Efficacy;
- Safety/tolerability;
- Cost;
- Potential for resistance[39]
.
The choice of prophylactic agents therefore needs to take into consideration local epidemiology to minimise the development of resistance. Antimicrobial prophylaxis agents used by GOSH are highlighted in Table 1[8],[9],[10],[11],[12],[13],[14],[15]
.
The pharmacist must be vigilant in monitoring blood culture sensitivities and count recovery, so appropriate antibiotics can be initiated, changed or stopped. For example:
- Prophylactic ciprofloxacin is stopped when the neutrophil count is >1.0 x 109/L;
- Prophylactic vancomycin is stopped when the neutrophil count is >0.2 x 109/L;
- Ambisome is switched to oral azoles when the child starts tolerating oral medicine;
- Phenoxymethylpenicillin and PCP prophylaxis is initiated at count recovery and all other antibiotics stopped[9]
.
Veno-occlusive disease
VOD is a serious complication of HSCT affecting about a third of at-risk patients. It is characterised by weight gain, fluid retention, ascites, peripheral oedema with third spacing, jaundice and painful hepatomegaly[18],[40]
. Other features include platelet consumption, coagulopathy and sodium retention[40]
.
Clinical criteria for diagnosing VOD:
- Bilirubin >34.2 μmol/L;
- Hepatomegaly or right upper quadrant pain;
- Weight gain of >5% from pre-transplant weight[18],[40]
.
Patients who are at risk of VOD include those:
- Receiving chemo conditioning with combination of either busulfan/cyclophosphamide or cyclophosphamide/TBI;
- With osteopetrosis (i.e. ‘stonebone’);
- Who have previously been treated with gemtuzumab (Mylotarg);
- With previous hepatomegaly;
- Aged under one year;
- With adrenoleukodystrophy;
- With previous abdominal radiation/TBI[18],[40]
.
For patients at risk of VOD, twice daily weight and girth measurements are monitored and recorded daily[9],[18]
. A strict fluid input/output is recorded if there are any signs or symptoms of VOD[41]
. All IV medicines are diluted into minimal volumes and glucose 5% is the preferred diluent wherever possible, instead of sodium chloride 0.9%, to restrict sodium and, therefore, water retention[18],[40]
.
Strict fluid management with diuretics (e.g. furosemide and spironolactone) and defibrotide is the main treatment for VOD[5],[6]
. Owing to the relatively high cost of defibrotide, a Bluteq form must be completed for funding approval by NHS England[41]
.
Graft versus host disease
GvHD is one of the most significant complications of HSCT, accounting for a substantial portion of transplant-related morbidity and mortality[42]
. GvHD is classified as acute when occurring within 100 days of transplant, and chronic if occurring beyond 100 days of transplant[26],[43]
.
Acute GvHD (aGvHD) classically targets the skin, gastro-intestinal (GI) tract and liver[26]
. The symptoms and signs of aGvHD are variable and include skin rash, anorexia, vomiting, diarrhoea, abdominal pain, haemotochezia and jaundice[26],[42]
. Symptoms are often associated with fever[26]
. The lungs may also be involved in aGvHD, resulting in respiratory distress and tachypnoea, with or without pulmonary infiltrates in the absence of infection, frequently around the time of neutrophil engraftment[26],[42]
. This is often referred to as ‘engraftment syndrome’ (around D+14)[26],[42]
.
Chronic GvHD is a late complication of allogeneic HSCT and is the leading cause of late non-relapse death[43]
. The prevalence varies from 25–80% in long-term survivors[43]
.
Patients with GvHD are often on systemic immunosuppression with corticosteroids, as well as ciclosporin[26],[43]
. Hence, they are at increased risk of infections and they will be on PCP prophylaxis, prophylactic antifungal, and aciclovir with weekly blood viral monitoring by polymerase chain reaction (PCR) for cytomegalovirus (CMV), Epstein-Barr virus and adenovirus[9]
.
Management and treatment of symptoms:
- Skin GvHD is treated with emollients and topical steroids. Topical and systemic antihistamines may also be required for itchy skin;
- Gut and liver GvHD are treated with systemic high-dose corticosteroids — up to 2mg/kg/day of IV methylprednisolone has been used;
- For steroid-refractory GvHD (defined as no improvement after two weeks of high dose corticosteroids) antibodies such as infliximab-basiliximab combination may be used;
- Extracorporeal photopheresis is an alternative treatment option that is becoming popular as a corticosteroid-sparing agent for steroid-refractory GvHD[44],[45],[46],[47]
.
Haemorrhagic cystitis
Haemorrhagic cystitis (HC) is a frequently observed complication after transplantation, resulting in prolonged hospital stays and occasionally death[48]
. It has a spectrum of manifestations that range from microscopic haematuria to severe haemorrhage with obstructive renal failure. The incidence of severe haemorrhagic cystitis is around 3 to 4% for both adult and paediatric paients[48]
.
Early HC is often associated with the use of cyclophosphamide[27]
. Acrolein, a metabolite of cyclophosphamide, is toxic to the transitional epithelium of the bladder mucosal tissue[27]
. All patients on cyclophosphamide are always on mesna, and increasing the dose of mesna may relieve early HC[27],[28]
. Radiation therapy has also been implicated in the aetiology of early HC[27]
. Later development of HC is commonly caused by viral infections affecting the urinary tract, most commonly polyoma viruses (e.g. BK or JC), less frequently adenovirus, and rarely CMV[48]
. Mesna has no role in HC caused by virus[48]
.
At GOSH, oestrogen patches are used to treat HC when hyperhydration is not effective and patients with severe bladder pain may require the use of oxybutynin for bladder spasms[27],[49]
.
The main clinical features of HC are suprapubic pain, dysuria, haematuria, urinary frequency, hydronephrosis and acute renal failure[48]
. In rare cases, HC may cause urinary tract obstruction[27],[50]
.
The principal management of HC caused by viral infection is to keep the bladder flushed by increasing fluid intake with hyperhydration (2L/m2/day), and promoting diuresis to maintain fluid balance and weight[48]
. Early referral to the urology team should be considered for patients not responding to hyperhydration and forced diuresis for bladder irrigation, if appropriate[48]
.
Posterior reversible encephalopathy syndrome
PRES is a recognised complication of HSCT. It is a syndrome which defines a collection of clinical symptoms and radiological signs that involve white — and sometimes grey — matter changes in particular areas in the brain[19]
. Incidence of PRES after adult bone marrow transplant is about 8%; this incidence is thought to be the same in paediatric patients[50],[51]
. It is associated with chemotherapy and immunosuppressants (e.g. ciclosporin, corticosteroids, tacrolimus and cyclophosphamide) used in the transplant settings[52]
.
Seizures are one of the most important symptoms of PRES. There are associated biochemical abnormalities and systemic observations that can precede the onset of the syndrome, and can help prevent or reduce its frequency[50]
. These include headaches, shakes, tremors, high blood pressure and low magnesium[19],[52]
. It is important to monitor plasma electrolytes to avoid hypomagnesemia and hypercalcaemia[19]
.
Hypertension is rigorously controlled with anti-hypertensives to maintain blood pressure[19]
.
PRES can usually be resolved once the offending agent is discontinued[52]
. When initiating azole antifungal therapy (e.g. fluconazole, itraconazole, posaconazole), ciclosporin dose is reduced by 50% and levels are monitored and adjusted accordingly. Azoles are CYP3 inhibitors and may increase ciclosporin levels to toxic range, which may precipitate PRES[52]
. PRES can also manifest when ciclosporin levels are within the normal range[19],[52],[53]
.
Thrombotic microangiopathy
There is wide variation in the reported incidence of TA-TMA owing to variability in the diagnostic criteria used in different publications[54]
. When uniform criteria are applied, the incidence is between 10–15%[55]
. TMA describes syndromes of refractory thrombocytopenia and haemolysis (red blood cell fragmentation) and increased lactate dehydrogenase associated with unexplained renal impairment and/or neurological impairment[20]
. Injury to the endothelial structure by high-dose chemotherapy, radiation, calcineurin inhibitors and cytokine production are involved in the pathophysiological mechanism[20]
.
Inflammatory cytokines released by GvHD-associated tissue injury have been linked to an increase in prothrombotic events, such as increases in tissue plasminogen activator and ultra-large von Willebrand factor assembly[20]
.
Management includes tight control of calcium and magnesium levels, as well as optimising antihypertensive treatment[54],[55]
. There are no established treatment options for TMA; however, calcineurin inhibitors (e.g. ciclosporin, tacrolimus) should be stopped at the first sign of the condition[54]
. Eculizumab (a humanised monoclonal antibody against complement component C5), and defibrotide, have been used as some novel treatment options, but data are very limited[54],[56]
.
Discharge planning and beyond
Despite the various complexities of HSCT, rigorous monitoring and management can ensure the child’s journey is as smooth as possible. As neutrophil count recovers, the tissue starts to heal and the pain from mucositis gradually improves, the child will usually start to drink and eat, and at this stage the clinical team can plan and discuss converting IV medications to oral in preparation for discharge home.
It is essential that both children and parents/carers are engaged as active partners in discussions about medicines, where the risks and benefits of treatment are considered. Working in partnership with families is a prerequisite for effective paediatric care, and may promote adherence and concordance to medication regimens at home[6]
. As part of this, the intrinsic values and beliefs of the family must be taken into account, as well as the practicalities of adhering to the proposed treatment and its impact on daily living[6]
.
The pharmacist should discuss alternative and appropriate formulations with the child and parents/carers, as well as ensuring that they understand the rationale and potential side effects of the drugs given. Considerations surrounding timings of medicines to suit family life, as well as them being pharmacologically acceptable, are an important part of the discharge planning process. Renal clearance, for example, is a concern following discharge, because it is not monitored as frequently, and patients and their parents/ carers are often more relaxed about fluid targets in the home setting.
Clinical staff need to be aware that the child is likely to be discharged on a complex regimen of multiple medications — even simple measures, such as reducing the volume of medication a child has to take by selecting a higher strength, can have an enormous impact on adherence to medications. Moreover, many children will be discharged with either an NGT or a PEG tube to facilitate medicine administration, and clinical staff need to be mindful that some preparations of medicines predispose the tube to block. This can be very distressing for both the child and parent/carer, and therefore it should be avoided[57]
. For example, the use of ciprofloxacin tablets, instead of ciprofloxacin suspension, can prevent blocking of the NG tube[57]
.
Prescribers need to be reminded that medicines must be appropriately and sensibly dose rounded to an easily measurable volume, for ease of administration[10]
. This should be part of medicines management throughout the admission, but it becomes particularly relevant during robust and timely discharge planning. Clear instructions and training in drug administration for parents and carers is vital and an essential part of appropriate safety netting. This is even more important for parents/carers who may not speak English or for those who struggle with literacy. For this cohort of carers, a colour-coded chart should be considered: ensuring adequate time is allocated for pre-discharge training and practice to avoid error and promote concordance[58]
.
Discharge medicines are prescribed and supplied at least three days in advance, so parents/carers can practice giving medicines under the supervision of the nurse to gain confidence, and can ask any questions on medicines. A parent/carer medicines chart is created to familiarise them with the volume and timing of medicines (see Figure 2).
The charts are generated by the CNS, with a final check by the pharmacist before discharge.
After discharge, the children are usually followed up in the outpatient clinic, on a weekly basis initially, and then less frequently, according to their progress. Children are reviewed until they are completely off their immunosuppression medications and then monitored for issues in the long-term follow-up clinic.
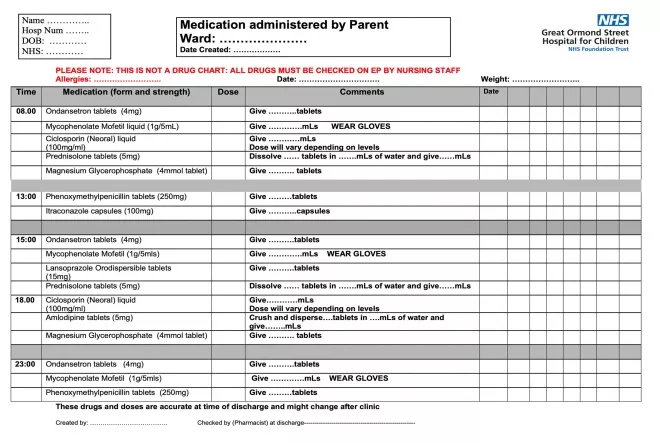
Figure 2: Sample of a parent medication chart
Best practice
Haematopoietic stem cell transplant (HSCT) is a complex procedure, with the potential for many adverse effects and complications. Therefore, the pharmacist should:
- Play an active role in the clinical management of these patients; for example, checking drug interactions owing to polypharmacy, and monitoring and advising when medicines are no longer required;
- Interact regularly with the medical team, the nurses on the wards, the children and parents/carers, because transplant demands a multidisciplinary team approach and the pharmacist is an integral part of that team.
Caring for paediatric HSCT patients is both complex and challenging but, ultimately, rewarding, as close relationships are developed with the children and families, who can experience lengthy admissions where the pharmacist’s input is invaluable.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
References
[1] NHS. Stem cell and bone marrow transplants. 2018. Available at: https://www.nhs.uk/conditions/stem-cell-transplant/ (accessed December 2020)
[2] Duncombe A. ABC of clinical haematology: bone marrow and stem cell transplantation. BMJ 1997;314:1179. doi: 10.1136/bmj.314.7088.1179
[3] American Cancer Society. Types of stem cell and bone marrow transplants. 2020. Available at: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/stem-cell-transplant/types-of-transplants.html (accessed December 2020)
[4] Anthony Nolan. Patients and families. 2014. Available at: https://www.anthonynolan.org/patients-and-families (accessed December 2020)
[5] Awortwe C, Makiwane M, Reuter H et al. Critical evaluation of causality assessment of herb–drug interactions in patients. Br J Clin Pharmacol 2018;84(4):679–693. PMID: 29363155
[6] National Institute for Health and Care Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical Guideline [CG76]. 2009. Available at: https://www.nice.org.uk/guidance/cg76/documents/medicines-concordance-guideline-consultation (accessed December 2020)
[7] Anthony Nolan. A young person’s guide to the stem cell transplant journey. 2017. Available at: https://www.anthonynolan.org/sites/default/files/1230PA%20teen%20and%20young%20persons%20guide.pdf (accessed December 2020)
[8] Groll AH, Castagnola E, Cesaro S et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 2014;15(8):e327–340. doi: 10.1016/S1470-2045(14)70017-8
[9] Great Ormond Street Hospital. Haemopoietic stem-cell protocol. Internal guidance [GOSH intranet]
[10] Grafter-Gvili A, Fraser A, Paul M & Leibovivi L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 2005;142:979–995. doi: 10.7326/0003-4819-142-12_Part_1-200506210-00008
[11] Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep 2000;49(RR-10):1–125,CE1–7. PMID: 11718124
[12] Erard V, Guthrie KA, Varley C et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood 2007;110(8):3071–3077. doi: 10.1182/blood-2007-03-077644
[13] European Society for Blood and Marrow Transplantation. EBMT Handbook. Chapter 13: Graft-versus-host disease. 2012. Available at: https://ebmtonline.forumservice.net/media/13/tex/content_alt/EBMT_Handbook2012_CHAP13.pdf (accessed December 2020)
[14] Martin PJ, Storer BE, Rowley SD et al. Evaluation of mycophenolate for initial treatment of chronic graft-versus-host disease. Blood 2009;113:5074–5082. doi: 10.1182/blood-2009-02-202937
[15] Engelhard D, Cordonnier C, Shaw PJ et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol 2002;117(2):444–450. doi: 10.1046/j.1365-2141.2002.03457.x
[16] European Society for Blood and Marrow Transplantation. EBMT Handbook. Chapter 11: Early complications after HSCT. Available at: https://ebmtonline.forumservice.net/media/11/tex/content_alt/EBMT_Handbook2012_CHAP11.pdf (accessed December 2020)
[17] Pinkerton R, Plowman P & Pieters R. Paediatric Oncology. 3rd Ed. Ann R Coll Surg Engl 2006;88(1):87. PMID: 1963643
[18] Dignan F, Wynn R, Hadzic N et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol 2013;163(4):444–457. doi: 10.1111/bjh.12558
[19] Roth C & Ferbert A. The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Neurology 2011;11:136–144. doi: 10.1136/practneurol-2011-000010
[20] Jodele S, Dandoy CE, Myers KC et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci 2016;54(2):181–190. doi: 10.1016/j.transci.2016.04.007
[21] European Society for Blood and Marrow Transplantation. EBMT Handbook. Chapter 10: Supportive care. 2012. Available at: https://ebmtonline.forumservice.net/media/10/tex/content_alt/EBMT_Handbook2012_CHAP10.pdf (accessed December 2020)
[22] Joint Formulary Committee. British National Formulary. [Online version] 79th ed. Ciclosporin. Side-effects. London: BMJ Group and Pharmaceutical Press; 2020. Available at: https://bnfc.nice.org.uk/drug/ciclosporin.html#sideEffects (accessed December 2020)
[23] Malhotra A, Maughan D, Ansell J et al. Choosing wisely in the UK: the Academy of Medical Royal Colleges’ initiative to reduce the harms of too much medicine. BMJ 2015;350:h2308. doi: 10.1136/bmj.h2308
[24] Swift A. Understanding the effect of pain and how the human body responds. Nursing Times 2018;114:3,22–26. Available at: https://www.nursingtimes.net/clinical-archive/pain-management/understanding-the-effect-of-pain-and-how-the-human-body-responds-26-02-2018/ (accessed December 2020)
[25] Gibson F, Cargill J, Allison J et al. Establishing content validity of the oral assessment guide in children and young people. Eur J Cancer 2006;42(12):1817–1825. doi: 10.1016/j.ejca.2006.02.018
[26] Dignan FL, Clark A, Amrolia P et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012;158(1):30–45. doi: 10.1111/j.1365-2141.2012.09129.x
[27] Miller LJ, Chandler SW & Ippoliti CM. Treatment of cyclophosphamide-induced hemorrhagic cystitis with prostaglandins — case report. Ann Pharmacother 1994;28(5)590–594. doi: 10.1177/106002809402800508
[28] Joint Formulary Committee. British National Formulary. [Online version] 79th ed. Mesna. Indications and dose. London: BMJ Group and Pharmaceutical Press; 2020. Available at: https://bnfc.nice.org.uk/drug/mesna.html#indicationsAndDoses (accessed December 2020)
[29] Schwartzberg LS. Chemotherapy-induced nausea and vomiting: clinician and patient. J Support Oncol 2007;5(2 Suppl 1):5–12. PMID: 17366928
[30] Dupius LL, Boodhan S, Sung L et al. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer 2011;57(2):191–198. doi: 10.1002/pbc.23114
[31] Macmillan Cancer Support. Nausea and vomiting. 2015. Available at: https://www.macmillan.org.uk/cancer-information-and-support/impacts-of-cancer/nausea-and-vomiting (accessed December 2020)
[32] Cancer Research UK. Music therapy. 2019. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/complementary-alternative-therapies/individual-therapies/music (accessed December 2020)
[33] European Society for Blood and Marrow Transplantation. EBMT Handbook. Chapter 10: Supportive care. 2012. Available at: https://ebmtonline.forumservice.net/media/10/tex/content_alt/EBMT_Handbook2012_CHAP10.pdf (accessed December 2020)
[34] Eilers J, Harris D, Henry K & Johnson LA. Evidence-based interventions for cancer treatment-related mucositis: putting evidence into practice. Clin J Oncol Nurs 2014;18(6):80–96. doi: 10.1188/14.CJON.S3.80-96
[35] Mouth care. Great Ormond Street Hospital. Internal guidance [GOSH intranet]
[36] McCulloh R, Hemsley J & Kelly P. Symptom management during chemotherapy. Paediatr Child Health 2014;24(4):166–171. doi: 10.1016/j.paed.2013.10.007
[37] Nikoletti S, Hyde S, Shaw T et al. Comparison of plain ice and flavoured ice for preventing oral mucositis associated with the use of 5 fluorouracil. J Clin Nurs 2005;14(6):750–753. doi: 10.1111/j.1365-2702.2005.01156.x
[38] European Society for Blood and Marrow Transplantation. EBMT Handbook. Chapter 10: Supportive care. 2012. Available at: https://ebmtonline.forumservice.net/media/10/tex/content_alt/EBMT_Handbook2012_CHAP10.pdf (accessed December 2020)
[39] Bock A, Cao Q, Ferrieri P et al. Bacteremia in blood or marrow transplantation patients: clinical risk factors for infection and emerging antibiotic resistance. Biol Blood Marrow Transplant 2013;19:102–108. doi: 10.1016/j.bbmt.2012.08.016
[40] Richardson PG, Ho VT, Cutler C et al. Hepatic veno occlusive disease after HSCT: novel insights to pathogenesis, current status of treatment, and future directions. Biol Blood Marrow Transplant 2013;19(1):S88–90. doi: 10.1016/j.bbmt.2012.10.023
[41] NHS England. Clinical commissioning policy: use of defibrotide in severe veno-occlusive disease following stem cell transplant. 2015. Available at: https://www.england.nhs.uk/wp-content/uploads/2018/07/Defibrotide-in-severe-veno-occlusive-disease.pdf (accessed December 2020)
[42] Ferrara J & Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol 2006;43(1):3–10. doi: 10.1053/j.seminhematol.2005.09.001
[43] Dignan FL, Amrolia P, Clark A et al. Diagnosis and management of chronic graftâ€versusâ€host disease. Br J Haematol 2012;158:46–61. doi: 10.1111/j.1365-2141.2012.09128.x
[44] Wolff D, Schleuning M, von Harsdorf S et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant 2011;17:1–17. doi: 10.1016/j.bbmt.2010.05.011
[45] Rao K, Rao A, Karlsson H et al. Improved survival and preserved antiviral responses after combination therapy with daclizumab and infliximab in steroid-refractory graft-versus-host disease. J Pediatr Hematol Oncol 2009;31:456–461. doi: 10.1097/MPH.0b013e31819daf60
[46] Schmidt-Hieber M, Fietz T, Knauf W et al. Efficacy of the interleukinâ€2 receptor antagonist basiliximab in steroidâ€refractory acute graftâ€versusâ€host disease. Br J Haematol 2005:130;568–574. doi: 10.1111/j.1365-2141.2005.05631.x
[47] Couriel DR, Hosing C, Saliba R et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907
[48] Harkensee C, Vasdev N, Gennery AR et al. Prevention and management of BK-virus associated HC in children following haematopoietic stem cell transplantation: a systematic review and evidence-based guidance for clinical management. Br J Haematol 2008:142(5):717–731. doi: 10.1111/j.1365-2141.2008.07254.x
[49] Rodriguez Luna JM, Teruel JL, Vallejo J et al. Control of massive hematuria in idiopathic hemorrhagic cystitis after administration of conjugated estrogen. J Urol 1992;148(5)1:1524–1525. doi: 10.1016/S0022-5347(17)36956-2
[50] Siegal D, Keller A, Xu W et al. Central nervous system complications after allogeneic hematopoietic stem cell transplantation: incidence, manifestations, and clinical significance. Biol Blood Marrow Transplant 2007;13(11):1369–1379. doi: 10.1016/j.bbmt.2007.07.013
[51] Uckan D, Cetin M, Yigitkanli I et al. Life-threatening neurological complications after bone marrow transplantation in children. Bone Marrow Transplant 2005;35(1):71–76. doi: 10.1038/sj.bmt.1704749
[52] Burnett MM, Hess CP, Roberts JP et al. Presentation of reversible posterior leukoencephalopathy syndrome in patients on calcineurin inhibitors. Clin Neurol Neurosurg 2010;112(10):886–891. doi: 10.1016/j.clineuro.2010.07.023
[53] Joint Formulary Committee. British National Formulary. [Online version] 79th ed. Interactions. Ciclosporin. London: BMJ Group and Pharmaceutical Press; 2020. Available at: https://bnfc.nice.org.uk/interaction/ciclosporin-2.html#bnf_i1603946460715 (accessed December 2020)
[54] Uderzo C, Jodele SC, Missiry ME et al. Transplant-associated thrombotic microangiopathy (TA-TMA) and consensus based diagnostic and therapeutic recommendations: which TA-TMA patients to treat and when? J Bone Marrow Res 2014;2(3):1–7. doi: 10.4172/2329-8820.1000152
[55] Uderzo C, Bonanomi S, Busca A et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplant 2006;82(5):638–644. doi: 10.1097/01.tp.0000230373.82376.46
[56] Kim SS, Patel M, Yum K & Keyzner A. Haemapoetic stem cell transplant-associated thrombotic micro angiopathy: review of pharmacological treatment options. Transfusion 2015;55(2):452–458. doi: 10.1111/trf.12859
[57] Handbook of Drug Administration via Enteral Feeding Tubes. Ciprofloxacin. 2015. Available at: https://www.pharmpress.com/files/docs/DrugAdmin3e_sample.pdf (accessed December 2020)
[58] Bhattacharya D. Indications for multi compartment compliance aids (MCA) — also known as monitored dosage systems (MDS) — provision. 2005. Available at: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support/toolkit/indications-for-mds.pdf (accessed December 2020)