BSIP PLATRIEZ/SCIENCE PHOTO LIBRARY
After reading this learning article, you should be able to:
- Understand the pre-transplantation evaluation process, including how patients are screened and how livers are allocated;
- Know how medications are managed prior to transplantation;
- Understand how immunosuppressants and other medications are managed following transplantation, and important counselling points for patient’s post-transplantation.
Since the first successful human liver transplant was performed by surgeon Thomas Starzl in 1967, liver transplantation (LT) has evolved to become a life-saving treatment[1]. The service is currently provided by seven adult and three paediatric centres in the UK. Waiting times are around four months; although, as demand continues to exceed supply of grafts, waiting list mortality remains at around 8%[2]. In the UK, there are usually around 400 people on the LT waiting list; however, this has risen to more than 600 during the course of the COVID-19 pandemic[2].
Patients are often very unwell prior to LT and management of their medications can be complex, impacted by deteriorating liver function and evolving complications, such as fluid overload and acute kidney injury[3]. Following successful LT, lifelong immunosuppression is required; the drugs used for this indication have a narrow therapeutic index, numerous potential side effects and drug-drug interactions with concomitant medications.
It is imperative that teams managing these patients have detailed knowledge of drugs commonly prescribed before and after LT. The role of pharmacists includes medicines optimisation pre- and post-transplantcon, provision of specialist medicines-related advice and patient counselling. This article outlines best practice for the safe and appropriate management of adult patients, focusing on medicines, at each stage of the transplant process.
Indications
Liver transplantation is indicated in four main groups of patients, each with different selection criteria based on factors defining the severity of disease and predicted benefit of LT.
Chronic liver disease is defined as liver cirrhosis with one or more features of hepatic decompensation (e.g. jaundice, ascites, hepatic encephalopathy or variceal bleeding) must be present with a UK Model for End-Stage Liver Disease (UKELD) score of ≥49 (calculated using serum sodium, creatinine, international normalised ratio and bilirubin levels, as described in Box 1). This score was derived from UK transplant data that demonstrated a score of ≥49 was the point at which one-year mortality from chronic liver disease exceeds that associated with complications arising from LT (about 9%)[4].
The most common causes of chronic liver disease for LT in the UK are fatty liver diseases (alcohol and non-alcohol related) and autoimmune liver diseases (primary sclerosing cholangitis, primary biliary cholangitis and autoimmune hepatitis)[5]. Rates of LT for hepatitis C have been rapidly falling since the introduction of direct-acting antiviral drugs, which are highly effective and well tolerated, even in patients with cirrhosis[6]. Owing to the rising obesity rate, non-alcoholic fatty liver disease (NAFLD) is expected to overtake alcohol as the leading indication for LT[7].
Box 1: UK Model for End-Stage Liver Disease score
Score components (derived from blood tests): International normalised ratio (INR), sodium, creatinine, bilirubin
Formula (via online calculators):
UK Model for End-Stage Liver Disease score = 5.395 × In (INR) + 1.485 × In (creatinine, μmol/L) + 3.13 × In(bilirubin, μmol/L) – 81.565 × In(sodium, mmol/L) + 435
Application: Minimal eligibility criteria for liver transplant (LT). Score ≥49 indicates the survival benefit from LT exceeds the risks from the operation.
ln = natural logarithm
Hepatocellular carcinoma (HCC) is a primary liver cancer arising from hepatocytes that most commonly involves cirrhosis, but can also occur in non-cirrhotic livers; NAFLD and the metabolic syndrome (the combination of diabetes, hypertension and obesity) are increasingly recognised as important risk factors[8]. Internationally, various systems (e.g. Milan Criteria, University of California San Francisco (UCSF), ‘Up-to-7’) are used for risk stratification of patients with HCC to determine their benefit from LT, while minimising risk of early cancer recurrence in the graft post transplantation[9]. Although most are based on the number and size of tumours in the liver, in the UK, an adapted form of the Milan Criteria are used: a single HCC ≤5cm or up to five HCC ≤3cm, or a single HCC 5–7cm that remains stable in size over six months[10]. These patients do not need a UKELD score ≥49 and will often have relatively well preserved liver function[9,11]. A pilot of LT in patients who do not meet these criteria but whose cancer has been “down staged” by locoregional treatment is currently ongoing[11,12]. Portal vein invasion or extra hepatic metastases are absolute contraindications to LT[11].
Acute liver failure is characterised by rapidly deteriorating liver function with jaundice, coagulopathy andhepatic encephalopathy, usually within 12 weeks of symptom onset[13]. The most common cause in the UK is paracetamol overdose[14]. Other causes include hepatitis A, hepatitis B, hepatitis E, autoimmune hepatitis and non-paracetamol drug-induced liver injury. These patients are usually very unwell with multi-organ failure and do not have underlying chronic liver disease. The LT listing criteria for acute liver failure in the UK are based on the King’s College Criteria, as summarised in Box 2[11]. For paracetamol overdose, a thorough psychiatric history is vital to compare the risks and benefits of LT versus supportive care. Patients with acute liver failure are prioritised for LT as the window for intervention can be narrow before progression to unrecoverable deterioration[11].
Box 2: King’s College Criteria for liver transplantation in acute liver failure
Acute liver failure owing to paracetamol overdose:
- Arterial pH <7.3 after resuscitation and >24 hours since ingestion;
- Lactate >3mmol/L, or
- Hepatic encephalopathy, serum creatinine >300µmol/L and INR>6.5
Acute liver failure owing to non-paracetamol causes:
- INR>6.5
- Three out of five of the following: unfavourable aetiology (e.g. drug-induced, autoimmune hepatitis), age <10 or >40 years, jaundice-encephalopathy time >7 days, bilirubin >300µmol/L, INR>3.5.
Variant syndromes include complications from chronic liver disease, leading to significantly impaired quality of life but without a qualifying UKELD score (e.g. refractory ascites, intractable pruritus or recurrent cholangitis), or rarer conditions, such as familial amyloidosis, polycystic liver disease and primary hyperlipidaemia[11]. These patients may wait a long time for grafts as their mortality is low compared to other indications[15].
Evaluation
Patients will undergo assessments to confirm indications for LT and assess fitness for major surgery. Typical investigations include comprehensive blood tests, cross-sectional imaging, an echocardiogram, lung function tests and an exercise test (e.g. cardiopulmonary exercise testing)[15]. The multidisciplinary team (MDT) includes hepatologists, surgeons, anaesthetists, drug and alcohol specialists, dieticians, transplant co-ordinators and pharmacists, who may play a role in addressing potential interactions with existing medications and compliance issues. The results of these investigations are presented in the MDT meeting, where consensus is sought to add the patient to the LT waiting list[15]. A limited set of investigations are required for an acutely deteriorating patient with acute liver failure, which focuses on excluding contraindications (e.g. metastatic malignancy) or other major comorbidities (e.g. heart disease).
Organ allocation
Until 2018, liver grafts were offered on a regional basis depending on the donor’s location. In this system, the local transplant unit was offered an available graft, and then matched it to a recipient on their list based on patient size, blood group and need (i.e. sickest patient)[16]. In 2018, the National Liver Offering Scheme was implemented for livers donated after brainstem death, based on the newly developed transplant benefit score. Comprising 21 factors relating to the recipient (e.g. age, gender, bilirubin, presence of ascites, time on the waiting list) and 7 factors relating to the donor (e.g. age, presence of diabetes and blood group) in an algorithm, a patient’s transplant benefit score integrates both their expected survival without a transplant (i.e. need for a transplant) and their expected survival following transplantation with the graft on offer (i.e. transplant utility)[16]. The scheme’s rationale was to improve equity of access to transplantation, reduce waiting list mortality and optimise the benefit achieved for each transplant. Grafts donated after brainstem death are now offered nationally on a named patient basis according to the calculated transplant benefit score[17]. Liver donations after circulatory death are usually more marginal grafts owing to prolonged periods of warm ischaemia before cardiac arrest occurs, but can increasingly be used with the aid of machine perfusion devices, and are still offered on a regional basis[17].
Medication management
Pre-transplant
Patients with liver cirrhosis have pathophysiological changes, such as alteration in hepatic blood flow, reduced protein synthesis and reduced/impaired drug metabolising enzymes, resulting in altered drug pharmacokinetics[18]. A study by Franz et al. showed that around 20% of drugs are incorrectly prescribed in these patients owing to incorrect dosing of medicines (e.g. sedatives/hypnotics, oral antidiabetics) and other factors, such as contraindicated drugs (e.g. non-steroidal anti-inflammatories). As a result, up to 30% of patients may experience adverse drug reactions (ADR)[19]. As the majority of these ADRs are preventable, it is imperative that drugs are carefully dosed and monitored in this patient cohort[18].
In the pre-transplant period, medicines optimisation is important to improve overall health and reduce potential complications. This may include prescribing additional drugs required for management of comorbidities (e.g. diabetes). These medications should be carefully evaluated for safety in liver impairment, with care taken for narrow therapeutic index drugs and where drug pharmacokinetics are strongly influenced by hepatic function[18]. An example of this is phenytoin, which is extensively metabolised by the liver and highly protein bound; therefore, concentrations may increase in liver impairment, hypoalbuminaemia and hyperbilirubinaemia meaning careful dose adjustments may be required, depending on drug levels[1,20]. As there is no universal guide on safety of use and/or dosage adjustments of drugs in liver impairment, it is important to consider individual patient parameters, comorbidities (e.g. renal impairment), severity of cirrhosis (e.g. model for end-stage liver disease (MELD) score), as well as pharmacokinetic and pharmacodynamic data. Useful resources include the summary of product characteristics and available trial data[18]. More recently, a Dutch website has been launched with safety and dosing recommendations for a limited number of drugs[18,21].
Another important consideration for medicines optimisation includes appropriate dose titrations of required drugs. Commonly used drugs in the treatment of complications from decompensated cirrhosis and considerations for their use are listed in Table 1.
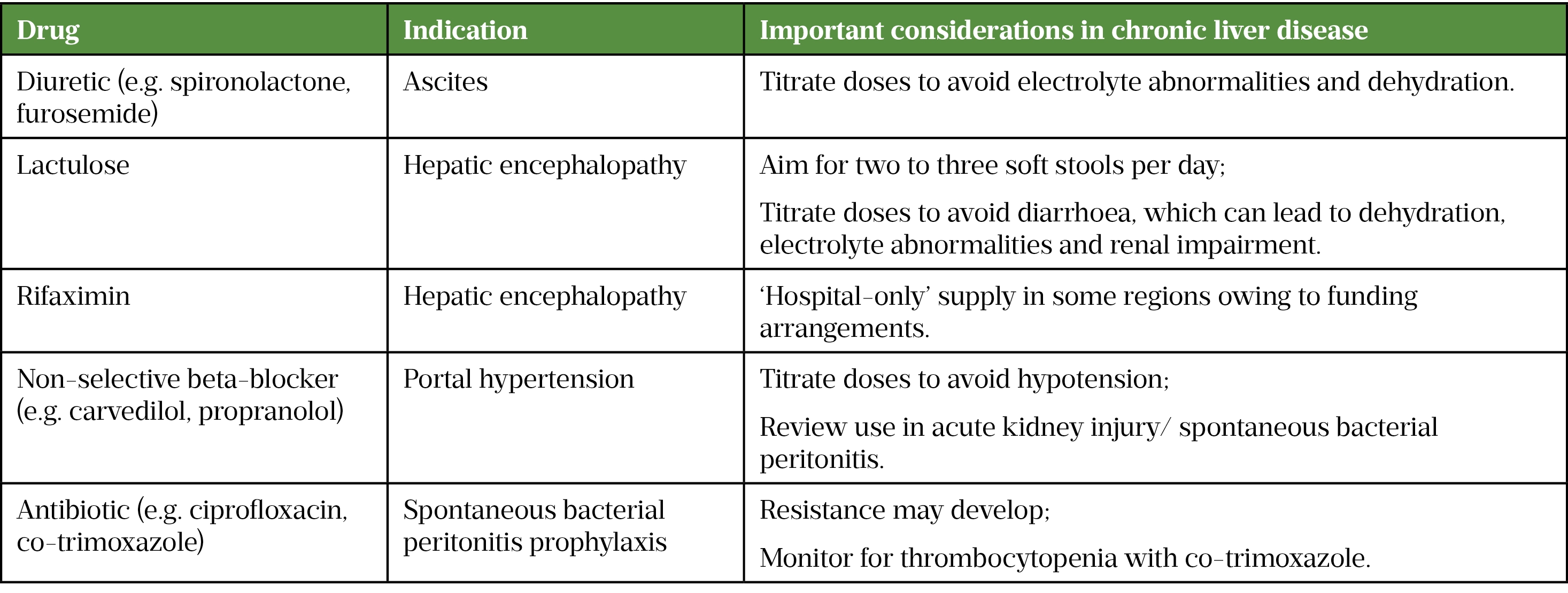
Deprescribing medication that has been started or continued inappropriately after an acute episode is an important part of medicines optimisation[22]. This may include proton pump inhibitors (PPIs) inappropriately prescribed for a variceal bleed. PPIs are not efficacious in the management of variceal bleeds and there is some evidence indicating that long-term PPI use in patients with cirrhosis is associated with increased mortality, further episodes of hepatic encephalopathy, spontaneous bacterial peritonitis and decompensation requiring hospital admission[23,24]. In patients with significant liver impairment, pain management should be carefully considered, with minimal doses of opiates as these may be largely metabolised and/or cleared via the liver, potentially leading to drug accumulation[25]. In addition, opiates can also cause constipation thereby increasing the risk of developing confusion orhepatic encephalopathy in cirrhotic patients[25]. Nephrotoxic drugs, such as non-steroidal anti-inflammatory drugs, should be avoided owing to prostaglandin inhibition, which can increase the risk of developing hepatorenal syndrome as well as the risk of bleeding[25].
Post-transplant
There will be several changes to patients’ medication regimens following transplant and this may continue from the immediate post-operative period to the weeks and months that follow.
Immunosuppressants
Class and mechanism of action
The common immunosuppressants used post liver transplant and their mechanism of action are listed in Table 2. This can also be viewed here.
![Table 2: Common immunosuppressant medication used post-liver transplantation[1,26–35]](https://pharmaceutical-journal.com/wp-content/uploads/2021/12/Table-2-Common-immunosuppressant-medication.png)
*Consult individual summaries of product characteristics for further details
Choice of immunosuppression regimen
The calcineurin inhibitors (CNIs) tacrolimus or ciclosporin make up the backbone of most immunosuppression regimens. Nearly all transplant patients will have a CNI prescribed, with tacrolimus being the CNI of choice, offering superior protection against graft loss and immunological failure, possibly owing in part to the vasospasm effect of ciclosporin and increased vascular complications[36]. The exact choice of immunosuppressant is heavily tailored to the individual patient, considering factors such as peri-operative renal impairment, liver disease aetiology, prior complications (e.g. graft rejection) and other side effects associated with each drug. A standard immunosuppression regimen immediately post-LT would be tacrolimus with IV methylprednisolone, switching to prednisolone once oral intake is established[37]. Tacrolimus dosing will be adjusted according to trough levels and prednisolone gradually weaned over 4–12 weeks. Some centres will add and continue a third agent (e.g. mycophenolate mofetil or azathioprine) if the underlying aetiology is immune-mediated or there is a prior history of rejection, although other centres will do this routinely in the early post-transplant period[37].
Renal-sparing regimens will be used if there is pre-operative renal impairment to reduce risk of further deterioration in renal function. This may involve halving the dose of tacrolimus and delaying its introduction beyond the usual day one start, in conjunction with the addition of basiliximab at day one and four.Mycophenolate mofetil or azathioprine may be added to permit a longer-term low-dose strategy with tacrolimus[38].
Sirolimus could be considered as an alternative, or in addition, to low-dose tacrolimus if the latter has prohibitive side effects, such as renal or metabolic toxicities[28]. However, it is best avoided in the early post-operative period owing to potential delayed wound healing and increased risk of early hepatic artery thrombosis[39].
Tacrolimus is preferred over ciclosporin but if neurotoxicity occurs a switch to ciclosporin may be considered[40].
Acute rejection is managed with pulse IV methylprednisolone 500mg–1g daily for 3 days, followed by augmentation of CNI dosing +/- an additional agent such as mycophenolate mofetil. Complex rejection may also require antithymocyte globulin (off label), given over seven to ten days, with dosing guided by the monitoring of blood lymphocyte levels[35]. The management of rejection is complex and is outside the scope of this article.
Administration and therapeutic drug monitoring
Although IV formulations of CNIs are available, these are very rarely used owing to increased risk of side effects, systemic toxicity and practicality issues, such as longer infusion times and complex calculations, making it a high-risk injectable medicine[41,42]. Instead, the oral route is preferred and various formulations exist, including liquid ciclosporin and tacrolimus capsules which may be administered via nasogastric tubing or sublingually (unlicensed)[26]. Tacrolimus is best taken on an empty stomach to achieve maximal absorption, but as this may not be practical in an inpatient setting, it should be taken in a consistent manner in relation to food. Patients are therefore counselled to take it at around the same time each day[26].
Drug levels of tacrolimus, ciclosporin and sirolimus require monitoring to ensure drug efficacy with minimal side effects. This will initially be daily in the immediate post-operative period, then weekly during early outpatient follow-up and gradually extended during the post-transplant period, according to the clinical situation (e.g. freedom from complications and good graft function would prompt reduced frequency of visits and therapeutic drug monitoring). Drug levels that are too high can cause toxicities (nephrotoxicity, neurotoxicity) and increased risk of infections, whereas levels that are too low can lead to graft rejection[26–28]. Trough levels for standard release formulations of tacrolimus and ciclosporin are checked 12 hours post dose, immediately prior to when the next dose is due[26,27]. On days when blood tests are due, the dose should be administered promptly after bloods have been taken, and patients counselled accordingly. The next day’s dose is taken at the usual time. If this test is in the afternoon, the timing since the last dose needs to be taken into account when the transplant physician interprets the trough level. In the immediate period post-LT, levels may be slightly higher (tacrolimus ~7-9ng/mL, ciclosporin ~150-200ng/L [local protocol]) and subsequently reduced three to six months following transplant[43]. Reference ranges may vary between patients depending on individual risk factors (e.g. renal function, graft function) and between transplanting centres; therefore, any changes to immunosuppression should only be made by the transplanting centre. Changes to medications, especially those with drug-drug interactions, will require closer monitoring of levels. Owing to the varying bioavailability between brands, tacrolimus and ciclosporin should always be prescribed by proprietary name. Changes in formulation may also affect drug levels; therefore, any switch (e.g. standard release to modified release) requires close therapeutic drug monitoring. Switching to modified-release once daily preparations of tacrolimus may be beneficial to improve compliance through reduced dosing requirements, or reduce side effects, such as renal dysfunction through lower overall tacrolimus exposure[44].
As tacrolimus, ciclosporin and sirolimus are substrates of the CYP3A group of enzymes, any other drug which is a substrate of, or affects the metabolism of, these enzymes will affect levels of these immunosuppressants[26–28]. It is critical to identify drug-drug interactions with newly prescribed medication, which may require more frequent therapeutic drug monitoring and/or a pre-emptive dose reduction of these agents. For example, commonly co-administered medications with powerfuldrug-drug interactions are macrolide antibiotics (e.g. clarithromycin) and azole antifungals, although the effects are not equal across class: for instance, fluconazole has a less potent interaction with tacrolimus than other azoles (e.g. ketoconazole) and may require less intensive monitoring of trough levels, especially at lower doses <200mg[45]. These drugs are potent CYP3A inhibitors and will increase immunosuppressant levels; therefore, their use may prompt closer monitoring of CNI or sirolimus levels and/or a dose reduction of CNI or sirolimus to avoid toxicity. Similarly, patients should also be advised to avoid grapefruit juice owing to its well-known effect on CYP450 enzymes. Likewise, herbal medications should be avoided owing to potential drug-drug interactions (e.g. St John’s wort is a potent CYP34A inducer and may reduce immunosuppression levels)[26–28,43].
Other common medications
Supportive medication is prescribed to prevent complications caused by side effects of immunosuppressive agents or to prevent infections in recipients, especially those with serological mismatch to the donor (e.g. recipient cytomegalovirus [CMV] immunoglobulin G [igG] negative/donor CMV IgG positive). Examples can be found in Table 3.
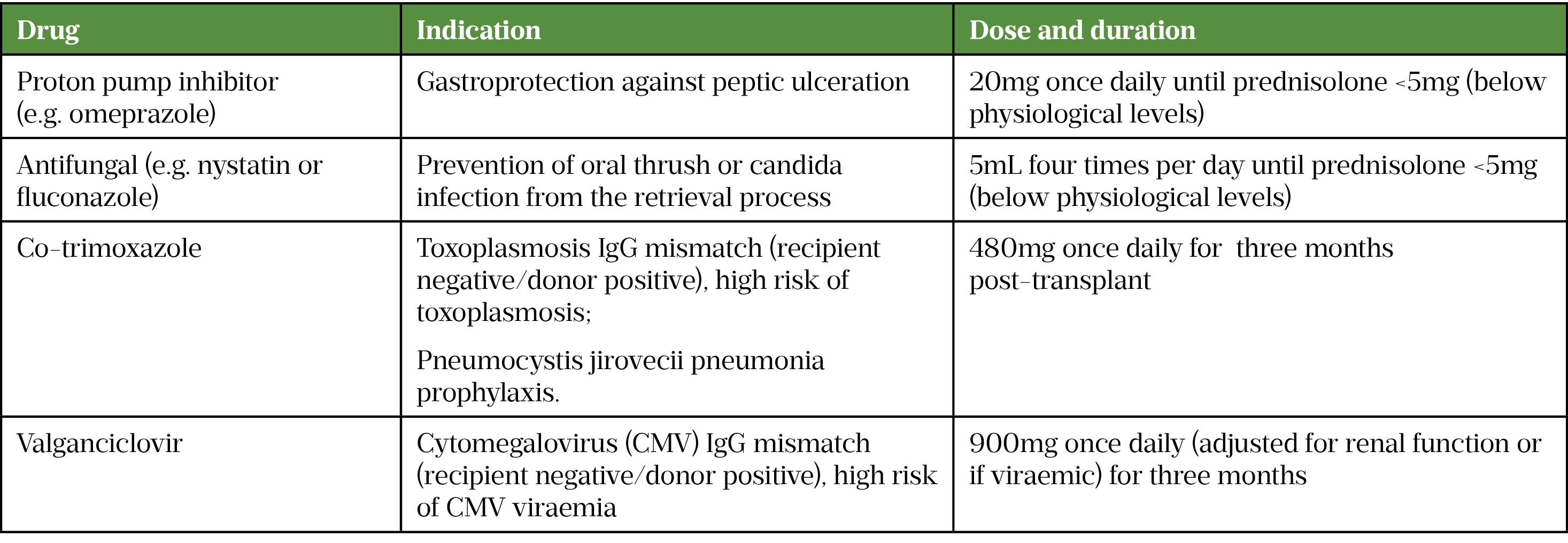
The indication, dosing and regimen is variable between centres. Please refer to the British National Formulary/relevant summary of product characteristics for information on side effects/contraindications
Antithrombotics, such as aspirin, low molecular-weight heparins or warfarin may be required to ensure patency of the hepatic artery and portal vein in patients who have surgical risk factors for thrombus development (e.g. complex hepatic artery reconstruction)[43]. There may be a role for direct oral anticoagulants (DOACs) in the future, but owing to limited experience and greater flexibility in the event of early post-operative complications, these are generally avoided at present. This risk is assessed by the surgeon at the time of operation, and the timing of anticoagulation introduction will consider various factors, such as platelet count, bleeding risk and prior history of portal vein thrombosis[32].
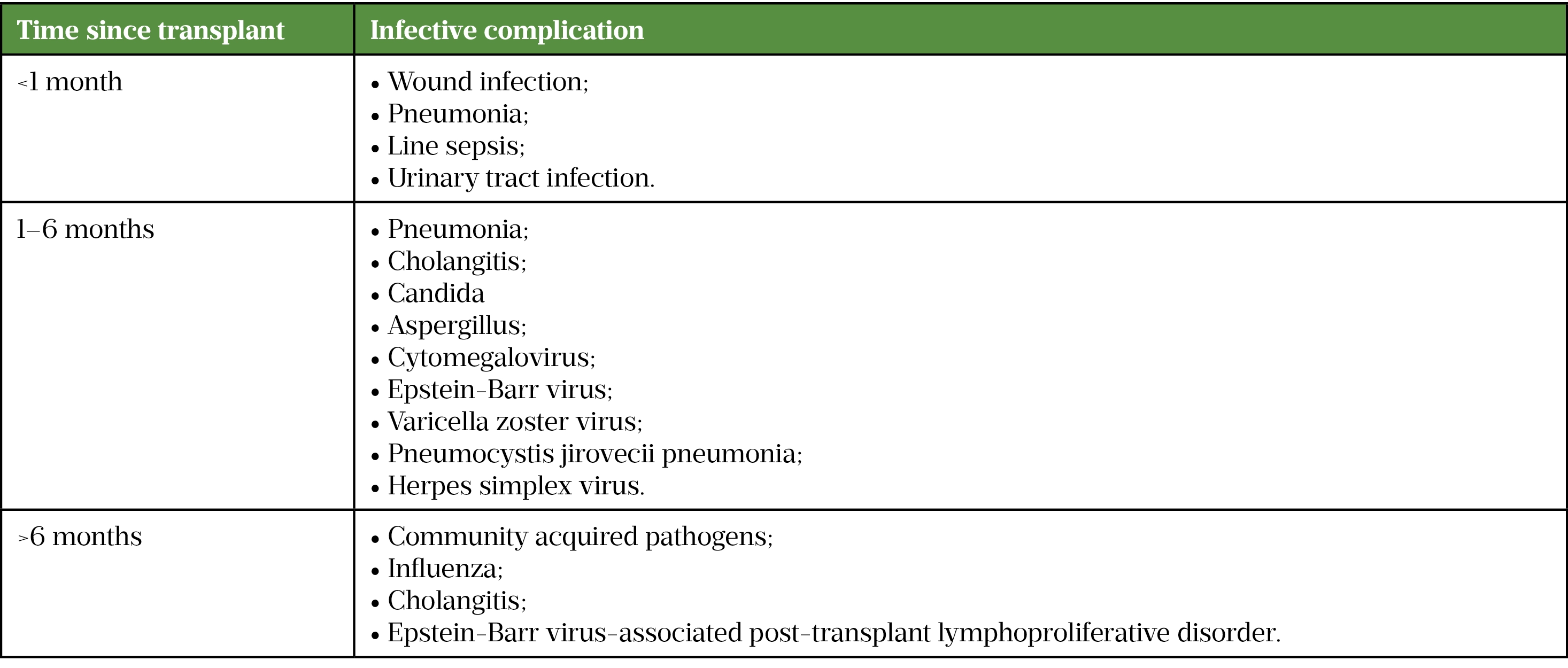
Infections
All patients are heavily immunosuppressed post-transplant to prevent rejection of the graft; this significantly increases the risk of infections[46]. Although infection may occur at any point, the time since transplant influences the risk profile for different types of infection[37]. Some antimicrobials and antifungals interact with immunosuppressants (e.g. clarithromycin and azole antifungals, discussed above) so careful consideration must be given to therapeutic drug monitoring and immunosuppressant levels during treatment of infective complications[43]. Table 4 summarises the types of infective complications commonly encountered at each stage post transplant.
Routine checks for CMV viraemia are usually performed at the outpatient visits. CMV can be asymptomatic, but may present with symptoms secondary to invasive tissue infections (e.g. diarrhoea with gastrointestinal mucosal disease or visual disturbance in ocular involvement); in cases of gastrointestinal disease, the diagnosis can be confirmed by the presence of inclusion bodies on mucosal biopsies at endoscopy. Treatment with oral valganciclovir or IV ganciclovir may be required if viraemic[47,48]. Some centres may use valganciclovir prophylaxis in certain settings (e.g. after antithymocyte globulin treatment for an episode of rejection)[47,48]. Dose modification of valganciclovir and ganciclovir may be required as renal impairment may be a complication post transplantation. A potential side of ganciclovir and valganciclovir is bone marrow suppression[50][51]; they should be discontinued if this occurs, and only restarted after recovery of bone marrow function, which may necessitate support with granulocyte colony stimulating factor[47,49,50].
In the later post-transplant period, Epstein-Barr virus is associated with the development of post-transplant lymphoproliferative disease and this should be considered in patients >6 months post-transplant who present with fever and weight loss[37].
Patient counselling
It is essential to counsel patients post-LT as there may be significant lifestyle changes involved. Adherence and compliance to medication is essential to prevent complications, such as graft rejection[51].
Important counselling points for the pharmacist to consider include:
- Ensuring patients have an adequate supply of medication at all times and know where this should be obtained, as medication supply routes may vary among transplant centres (e.g. homecare, outpatient pharmacy, GP);
- Ensuring changes to regular medication are communicated to the GP on discharge;
- Ensuring patients are informed about relevant drug-drug interactions, including with over-the-counter medication, and are able to recognise signs of infection (e.g. high fever, cough, shortness of breath, diarrhoea and vomiting) and have a point of contact at the transplant centre in case of queries;
- Ensuring that patients who are planning to start a family discuss this with their transplant consultant to ensure it is safe;
- Advising patients that when travelling, they check that recommended vaccinations and travel medication are compatible with their immunosuppression regimen, and to ensure they take relevant paperwork and adequate supply of their medication;
- Advising patients to always wear high factor sunscreen and protective clothing, owing to the increased risk of skin cancers caused by immunosuppression[26,27].
Follow-up
Patients will be seen weekly in the transplant clinic for the first one to two months post-discharge for blood tests and clinical review. The visit interval will gradually increase according to their progress. Vigilance is required, with timely response to any clinical developments, such as abnormalities in blood tests, as any delay can result in rapid clinical deterioration (e.g. graft loss) if not acted upon.
Some centres operate a ‘hub-and-spoke’ service with regional centres, who have the expertise to prepare potential liver transplant candidates and take over the post-transplant care of their patients, providing a more local and convenient service, with support from the ‘hub’ centre. An example of this is the link between the Royal Free Hospital in London with the Portsmouth Liver Centre on the south coast of England.
Conclusion
Pre- and post-transplant care can be complex and requires the involvement of various healthcare professionals. Infections may pose a significant risk post transplant so patients should be carefully monitored. Medication must be carefully considered in view of hepatic function pre-transplant and for drug-drug interactions and therapeutic drug monitoring post-transplant. Finally, patient education as well as clear communication and coordinated care between the healthcare professionals involved are imperative in ensuring positive patient outcomes and experience.
- 1STARZL TE, MARCHIORO TL, PORTER KA, et al. HOMOTRANSPLANTATION OF THE LIVER. Transplantation. 1967;5:790–803. doi:10.1097/00007890-196707001-00003
- 2Annual report on liver transplantation: report for 2019/2020 . NHS Blood and Transplant. 2020.https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19867/nhsbt-liver-transplant-report-1920.pdf (accessed Dec 2021).
- 3Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. The Lancet. 2014;383:1749–61. doi:10.1016/s0140-6736(14)60121-5
- 4Barber K, Madden S, Allen J, et al. Elective Liver Transplant List Mortality: Development of a United Kingdom End-Stage Liver Disease Score. Transplantation. 2011;92:469–76. doi:10.1097/tp.0b013e318225db4d
- 5Organ donation and transplantation activity report 2018/19. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16537/organ-donation-and-transplantation-activity-report-2018-2019.pdf. 2019.https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16537/organ-donation-and-transplantation-activity-report-2018-2019.pdf (accessed Dec 2021).
- 6Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. Journal of Hepatology. 2018;69:810–7. doi:10.1016/j.jhep.2018.06.010
- 7Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology. 2018;70:487–95. doi:10.1002/hep.29473
- 8Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clinical Gastroenterology and Hepatology. 2016;14:124-131.e1. doi:10.1016/j.cgh.2015.07.019
- 9Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2018;69:182–236. doi:10.1016/j.jhep.2018.03.019
- 10Mazzaferro V, Regalia E, Doci R, et al. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med. 1996;334:693–700. doi:10.1056/nejm199603143341104
- 11Liver transplantation: selection criteria and recipient registration. NHS Blood and Transplant Organ Donation and Transplantation. 2017.http://odt.nhs.uk/pdf/liver_selection_policy.pdf (accessed Dec 2021).
- 12Duvoux C, Roudot–Thoraval F, Decaens T, et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology. 2012;143:986-994.e3. doi:10.1053/j.gastro.2012.05.052
- 13Bernal W, Wendon J. Acute Liver Failure. N Engl J Med. 2013;369:2525–34. doi:10.1056/nejmra1208937
- 14Marudanayagam R, Shanmugam V, Gunson B, et al. Aetiology and outcome of acute liver failure. HPB. 2009;11:429–34. doi:10.1111/j.1477-2574.2009.00086.x
- 15Millson C, Considine A, Cramp ME, et al. Adult liver transplantation: A UK clinical guideline – part 1: pre-operation. Frontline Gastroenterol. 2020;11:375–84. doi:10.1136/flgastro-2019-101215
- 16The new National Liver Offering Scheme: What’s changing and how it will affect you. NHS Blood and Transplant. 2018.https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/7892/national-liver-offering-roadshow-presentation.pdf (accessed Dec 2021).
- 17Deceased Donor Liver Distribution and Allocation. NHS Blood and Transplant. 2019.https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/15920/liver-allocation-policy-pol-196.pdf (accessed Dec 2021).
- 18Weersink RA, Bouma M, Burger DM, et al. Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion. BMJ Open. 2016;6:e012991. doi:10.1136/bmjopen-2016-012991
- 19Franz CC, Hildbrand C, Born C, et al. Dose adjustment in patients with liver cirrhosis: impact on adverse drug reactions and hospitalizations. Eur J Clin Pharmacol. 2013;69:1565–73. doi:10.1007/s00228-013-1502-z
- 20Phenytoin Sodium Flynn Hard Capsules 100mg. Electronic medicines compendium. 2019.https://www.medicines.org.uk/emc/product/7558/smpc#gref (accessed Dec 2021).
- 21Weersink RA, Bouma M, Burger DM, et al. Evidence-Based Recommendations to Improve the Safe Use of Drugs in Patients with Liver Cirrhosis. Drug Saf. 2018;41:603–13. doi:10.1007/s40264-017-0635-x
- 22Duerden M, Payne R, Avery T. Polypharmacy and medicines optimisation: making it safe and sound. The King’s Fund. 2013.https://www.kingsfund.org.uk/publications/polypharmacy-and-medicines-optimisation (accessed Dec 2021).
- 23Angeli P, Bernardi M, Villanueva C, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology. 2018;69:406–60. doi:10.1016/j.jhep.2018.03.024
- 24Janka T, Tornai T, Borbély B, et al. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol 2020;32:257–64. doi:10.1097/MEG.0000000000001499
- 25Chandok N, Watt KDS. Pain Management in the Cirrhotic Patient: The Clinical Challenge. Mayo Clinic Proceedings. 2010;85:451–8. doi:10.4065/mcp.2009.0534
- 26Adoport 0.5 mg Hard Capsules. Electric medicines compendium. 2021.https://www.medicines.org.uk/emc/product/585/smpc#gref (accessed Dec 2021).
- 27Neoral Soft Gelatin Capsules. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/1034/smpc#gref (accessed Dec 2021).
- 28Rapamune 2mg coated tablets. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/medicine/5747#gref (accessed Dec 2021).
- 29Pevanti tablets. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/1741/smpc#gref (accessed Dec 2021).
- 30Solu-Medrone 1 gram Powder for Injection. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/3066/smpc#gref (accessed Dec 2021).
- 31Azapress Tablets 50mg. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/9624/smpc#gref (accessed Dec 2021).
- 32EASL Clinical Practice Guidelines: Liver transplantation. Journal of Hepatology. 2016;64:433–85. doi:10.1016/j.jhep.2015.10.006
- 33Cellcept 250mg Capsules. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/1102/smpc#gref (accessed Dec 2021).
- 34Simulect 20mg powder and solvent for solution for injection or infusion. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/7834/smpc#gref (accessed Dec 2021).
- 35Thymoglobuline 25 mg powder for solution for infusion. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/6238/smpc#gref (accessed Dec 2021).
- 36Haddad E, McAlister V, Renouf E, et al. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database of Systematic Reviews. 2006. doi:10.1002/14651858.cd005161.pub2
- 37Millson C, Considine A, Cramp ME, et al. Adult liver transplantation: UK clinical guideline – part 2: surgery and post-operation. Frontline Gastroenterol. 2020;11:385–96. doi:10.1136/flgastro-2019-101216
- 38Duvoux C, Pageaux GP. Immunosuppression in liver transplant recipients with renal impairment. Journal of Hepatology. 2011;54:1041–54. doi:10.1016/j.jhep.2010.12.001
- 39Massoud O, Wiesner RH. The use of Sirolimus should be restricted in liver transplantation. Journal of Hepatology. 2012;56:288–90. doi:10.1016/j.jhep.2011.06.012
- 40Tavabie OD, Colwill M, Adamson R, et al. A ‘real-world’ analysis of risk factors for post liver transplant delirium and the effect on length of stay. European Journal of Gastroenterology & Hepatology. 2020;32:1373–80. doi:10.1097/meg.0000000000001661
- 41Farouk SS, Rein JL. The Many Faces of Calcineurin Inhibitor Toxicity—What the FK? Advances in Chronic Kidney Disease. 2020;27:56–66. doi:10.1053/j.ackd.2019.08.006
- 42Specimen High Risk Injectable Medicines List. Specialist Pharmacy Service. 2016.https://www.sps.nhs.uk/articles/specimen-high-risk-injectable-medicines-list/ (accessed Dec 2021).
- 43Neuberger J, Ferguson J, Newsome PN, et al., editors. Liver Transplantation. Wiley 2021. doi:10.1002/9781119634010
- 44Lim TY, McPhail MJ, Shah A, et al. Sequential Cohort Analysis After Liver Transplantation Shows de Novo Extended Release Tacrolimus Is Safe, Efficacious, and Minimizes Renal Dysfunction. Transplantation Direct. 2020;6:e528. doi:10.1097/txd.0000000000000970
- 45Saad AH, DePestel DD, Carver PL. Factors Influencing the Magnitude and Clinical Significance of Drug Interactions Between Azole Antifungals and Select Immunosuppressants. Pharmacotherapy. 2006;26:1730–44. doi:10.1592/phco.26.12.1730
- 46Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856–79. doi:10.1111/ajt.14208
- 47The Prevention and Management of CMV Disease after Solid Organ Transplantation. British Transplantation Society. 2015.https://bts.org.uk/wp-content/uploads/2016/09/14_BTS_CMV_3RDE-1.pdf (accessed Dec 2021).
- 48Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33. doi:10.1111/ctr.13512
- 49Valcyte 450 mg Film-Coated Tablets. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/medicine/9315#gref (accessed Dec 2021).
- 50Cymevene 500mg powder for concentrate for solution for infusion. Electronic medicines compendium. 2021.https://www.medicines.org.uk/emc/product/10242/smpc#gref (accessed Dec 2021).
- 51Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17:760–70. doi:10.1002/lt.22294