DU CANE MEDICAL IMAGING LTD / SCIENCE PHOTO LIBRARY
Heart failure is a long-term condition that affects around 550,000 people in the UK, with more than 60,000 new cases diagnosed each year[1]
. It is defined by the National Institute for Health and Care Excellence (NICE) as “a common complex clinical syndrome of symptoms and signs caused by impairment of the heart’s action as a pump supporting the circulation caused by structural or functional abnormalities of the heart”[2]
. Despite advances in treatment for heart failure, mortality rates remain high, with age being an independent risk factor for mortality; patients aged under 75 years have significantly lower inpatient mortality compared with those aged over 75 years (4.4% versus 11.2%, respectively)[3]
. Hospital admissions for heart failure are frequent and are the leading cause of admission in patients aged over 65 years[4]
.
Frailty in people with heart failure has been extensively studied. The prevalence of patients with both conditions is common[5],[6],[7]
and associated with worse outcomes, including mortality[7],[8],[9],[10],[11],[12]
, increased hospital admissions[7],[8],[10],[13]
and reduced quality of life[14],[15],[16]
.
Frailty can be described as a complex interplay of health and illness, attitudes, resources and dependence on others, which leads to a decreased ability to withstand illness without loss of function[17]
. The body gradually loses its inbuilt reserves, putting patients at greater risk of adverse outcomes after seemingly minor events[18]
. Many people with long-term conditions (including heart failure) are frail, which can often be masked when the focus is on the other disease-based long-term conditions[18]
.
There are two well-known models for identifying frailty: the phenotype model of physical frailty, based on five pre-defined criteria (i.e. weight loss, exhaustion, slow gait speed, poor hand grip strength and sedentary behaviour), which was proposed and validated by Fried et
al.
[19]
; and the frailty index cumulative deficit model that focuses on physiological, medical and functional deficits to diagnose frailty, as proposed and validated by Rockwood et al.[20]
. Despite several validated tools for identifying patients with frailty[20],[21],[22] being available, none are currently validated within a heart failure population[23]
. The prevalence of frailty in the general older population is around 10%[18]
; prevalence rises with increasing age from 6.5% in those aged 60–69 years to around 30.0% in those aged 80–89 years, and 65.0% in those aged over 90 years[24]
. Up to 76.0% of patients with heart failure who are admitted to hospital are frail[7]
.
Both heart failure and frailty are linked with an ageing population, and prevalence is likely to increase over time. There are currently more than 3 million people aged over 80 years in the UK and the group aged over 85 years is the fastest growing population group[25]
. In general, older people are admitted to hospital more frequently, have longer lengths of stay and occupy more bed days than other patient groups in acute hospitals[26]
, which exposes them to avoidable harm[27]
and reduces their independence[26]
. Frailty contributes to increasing healthcare costs in care settings, such as hospital inpatients, hospital outpatients and nursing homes[28]
. In the 2016/2017 National Heart Failure Audit in the UK, the average age at first admission to hospital with heart failure was 78 years[3]
, with 67% of patients aged 75 years or over and around 33% aged over 85 years[3]
.
Owing to an ageing population and the increasing age of people with heart failure in general, treatment is becoming more complex. More than 60% of people with heart failure have at least three comorbidities[29]
and take an average of ten medicines[30]
, predisposing them to adverse drug events and poorer outcomes[31],[32],[33]
. Frail patients respond differently to both illness and medicines, often taking longer to recover with increased dependence needs and increased susceptibility to adverse drug reactions[34]
. Patients with heart failure often have episodes of decompensation within their disease trajectory, which is likely to have a significant impact on a patient with a coexisting frailty diagnosis, as is the prescription of complex treatment regimens.
Despite extensive research defining prevalence and outcomes for frail patients in a heart failure population, there is little in-depth knowledge about the treatment and management of this cohort to improve outcomes. This article will discuss the diagnostic criteria and the treatment options available, including the pharmacokinetic and pharmacodynamic considerations in older, frail people with heart failure.
Diagnosis
NICE describes the diagnostic process of heart failure as including: taking a full medical history, an examination, an electrocardiogram, a chest X-ray, blood tests (including N-terminal prohormone of brain natriuretic peptide [NT-proBNP] to exclude other disorders that may present in a similar manner), and an echocardiogram (ECHO)[2]
.
It can be more difficult to diagnose heart failure in older and frail people owing to the overlap in signs and symptoms (see Figure 1), compounded by multimorbidity and complex medicine regimens. Frailty is linked with poor mobility, difficulty doing everyday activities and generally ‘slowing down’ — not dissimilar to heart failure.
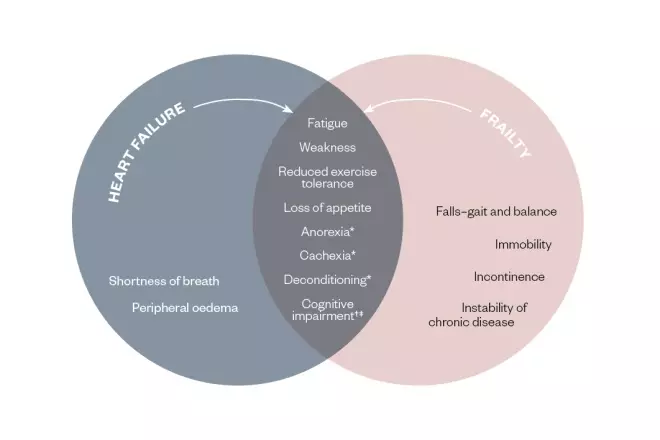
Figure 1: Comparison of symptoms in frailty and heart failure
*In advanced heart failure
†In decompensated heart failure
‡Source: ÄŒelutkienÄ— J, VaitkeviÄius A, JakÅ¡tienÄ— S & Jatužis D. Expert opinion — cognitive decline in heart failure: more attention is needed. Card Fail Rev 2016;2(2):106–109. doi: 10.15420/cfr.2016:19:2
There is an overlap between clinical symptoms for heart failure and frailty, making differentiation between the two conditions challenging
A detailed history and recognition of signs and symptoms are important in the diagnosis of heart failure, more so in an older, frail population. Older patients have often adapted their lifestyle to compensate for many of the symptoms and often attribute those symptoms to age.
Signs and symptoms
Shortness of breath is a common symptom spanning several comorbid conditions. As patients age, they become more accepting of symptoms such as reduced exercise tolerance. In many cases, patients have adapted their lifestyle to avoid becoming breathless and it is only when their breathing has declined significantly beyond what they would expect for their age, that they notice there is a problem.
In the 2016/2017 National Heart Failure Audit in the UK, 79% of patients admitted to hospital owing to heart failure had New York Heart Association (NYHA) Class III–IV breathlessness[3]
,which is defined as either breathlessness on minimal exertion, such as washing and dressing, or breathlessness at rest.
Paroxysmal nocturnal dyspnoea and orthopnoea are typical clinical symptoms of heart failure[35]
. As people age, sleeping patterns often change. Frequent waking owing to nocturia or sleeping in recliners or hospital beds that change position for comfort and ease of use can mean that older people do not identify the symptom burden. Tiredness and lethargy are common in older patients across several long-term conditions and becomes less noticeable as a specific symptom of heart failure.
Oedema also becomes less specific with age. As patients become less mobile, oedema can be an issue. In addition, older, frail patients with a reduced nutritional intake can have low albumin levels, which can lead to oedema. Comorbid conditions, such as chronic kidney disease, cor pulmonale (right ventricular enlargement secondary to a lung disorder) and cirrhosis of the liver, can also cause varying degrees of oedema. Furthermore, oedema is an adverse drug reaction of many medicines, clouding the diagnostic picture (see Table 1).
Similarly, pulmonary crackles are less specific in older people, sometimes owing to the co-existence of lung disease.
| Drug type | Very common (>1/10) | Common (1/10 to 1/100) | Uncommon (1/100 to 1/1,000) | Rare (1/1,000 to 1/10,000) | Very rare (>1/10,000) |
|---|---|---|---|---|---|
| Non-steroidal anti-inflammatory drugs | Naproxen | Ibuprofen Diclofenac | |||
| Antihypertensives | Isosorbide dinitrate Felodipine | Amlodipine Doxazosin Prazosin | Moxonidine Lercanidipine | ||
| Corticosteroids | Prednisolone Fludrocortisone Dexamethasone | ||||
| Antidiabetics | Vildagliptin Pioglitazone | ||||
| Chemotherapy agents | Cisplatin Pemetrexed Trastuzumab Docetaxel | Capecitabine | Cyclophosphamide | ||
| Antipsychotics | Olanzapine Risperidone Quetiapine | Haloperidol | |||
| Hormone therapy | Oestrogen | ||||
| Insulins | Degludec Detemir | Glargine | |||
| Anti-epileptics | Carbamazepine Gabapentin Pregabalin | Valproate | |||
| Anti-androgens | Bicalutamide | ||||
| Parkinson’s disease medication | Amantadine | Cabergoline Ropinirole Selegiline Amantadine | Levodopa | ||
| Antidepressants | Mirtazapine Phenelzine | Moclobemide Citalopram Sertraline | Imipramine Paroxetine | ||
| Proton pump inhibitors | Lansoprazole | Omeprazole | Pantoprazole | ||
| Bisphosphonates | Alendronate | Zoledronic acid | Ibandronate | Pamidronate | |
| Antibiotics | Doxycycline | Azithromycin | |||
| Statins | Atorvastatin | ||||
| Note: This list is not exhaustive; information taken from side effects listed in the BNF and summaries of product characteristics | |||||
Investigations
Chest X-rays have low specificity and sensitivity, which impacts their use in aiding heart failure diagnosis, and the common presence of co-existing lung disease in older people makes the interpretation of pulmonary oedema more difficult.
Measuring NT-proBNP levels is a useful way of ruling out heart failure as a diagnosis. Levels less than 400 nanograms/L make a diagnosis of heart failure less likely[2]
. Conversely, a high NT-proBNP level does not necessarily indicate heart failure as there are many non-cardiac causes for a raised level (see Box)[36],[37]
. A raised NT-proBNP level in an older patient should be investigated; however, consideration should also be given to alternative reasons for a raised result, such as increasing age and multimorbidity.
Box: Non-heart failure causes for raised N-terminal prohormone of brain natriuretic peptide levels
- Age;
- Atrial fibrillation/cardiac dysrhythmia;
- Chemotherapy/cancer;
- Chronic lung disease;
- Critical illness;
- Hyperthyroid;
- Ischaemic and haemorrhagic stroke;
- Liver cirrhosis;
- Obstructive sleep apnoea;
- Pneumonia;
- Pulmonary hypertension;
- Kidney impairment;
- Pulmonary embolism;
- Sepsis.
Source: QJM
[36]
An ECHO is the gold standard for diagnostics in heart failure. It is worth noting that, with ageing, there is inevitably a chance of wear and tear on the structural components of the heart, but this needs to be matched with clinical evidence of heart failure. Carrying out an ECHO allows for detailed imaging of the heart’s function and for specific measurements to be taken to calculate ejection fraction (EF). Heart failure with a reduced EF (HFREF) is defined as having an EF of less than 40%[2],[35]
. Heart failure with a preserved EF (HFPEF) is more of a diagnostic challenge without general consensus on diagnostic criteria, although an EF of greater than 50% with signs and symptoms is a common theme[2],[35]
. The evidence base for treatment of HFREF and HFPEF also differs.
Treatment
The use of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta blockers (BBs) and mineralocorticoid receptor antagonists (MRAs) have proved hugely beneficial in reducing mortality and morbidity and improving quality of life[2],[35]
. These medicines are the cornerstone of management in HFREF; albeit in a typically younger population than that seen today. The evidence base for the treatment of HFPEF is not as robust and, so far, clinical trials investigating ACEIs, BBs and MRAs have not shown benefit in long-term outcomes. The common treatment for both conditions is that of symptom management with diuretic therapy.
Evidence for the treatment of older people with heart failure is lacking. Clinical trials spanning almost 30 years and including almost 85,000 patients have an average participant age of 64 years, not typical of today’s population (see Table 2). It is thought that the reasoning behind this is that older people with heart failure are more likely to have a diagnosis of HFPEF linked with comorbidity rather than HFREF[35],[38]
. However, it is also worth considering that in the 1980s, when heart failure trials began, average life expectancy was 75 years with only 30% of the population surviving to over 80 years, which is significantly lower than that of recent years. In 2015, average life expectancy was 87 years with almost 70% of the population living into their 80s[39]
.
| Year | Trial | Medicine(s) | Number of participants (n) | Average age (years) | Exclusions |
|---|---|---|---|---|---|
| 1986 | Vasodilator Heart Failure Trial (V-HeFT) | Isosorbide dinitrate/hydralazine | 5,010 | 58 | Age >75 years |
| 1987 | Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) | Enalapril | 253 | 71 | |
| 1991 | Studies of Left Ventricular Dysfunction (SOLVD) | Enalapril | 2,569 | 61 | Age >80 years |
| 1992 | Survival and Ventricular Enlargement (SAVE) trial | Captopril | 2,231 | 59 | |
| 1996 | US Carvedilol Heart Failure Study Group | Carvedilol | 1,094 | 58 | |
| 1997 | Digitalis Investigation Group (DIG) Trial | Digoxin | 6,800 | 63 | |
| 1999 | Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) | Metoprolol | 3,991 | 64 | Age >80 years |
| Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) | Bisoprolol | 2,647 | 61 | Age >80 years | |
| Randomised Aldactone Evaluation Study (RALES) | Spironolactone | 1,663 | 65 | ||
| 2001 | The Valsartan Heart Failure Trial (Val-HeFT) | Valsartan | 5,010 | 63 | |
| Carvedilol Post- Infarct Survival Control in LV Dysfunction (CAPRICORN) study | Carvedilol | 1,959 | 63 | ||
| The Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study | Carvedilol | 2,289 | 63 | ||
| 2003 | Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM)-Added trial | Candesartan | 2,548 | 64 | 15% of the study population were aged over 75 years |
| Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM)-Alternative trial | Candesartan | 2,028 | 67 | ||
| Valsartan In Acute Myocardial Infarction (VALIANT) trial | Valsartan | 14,703 | 65 | ||
| Carvedilol Or Metoprolol European Trial (COMET) | Carvedilol vs. metoprolol | 3,029 | 62 | ||
| Epleronone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial | Eplerenone | 6,642 | 64 | ||
| 2005 | Randomised trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) | Nebivolol | 2,128 | 76 | Age <70 years |
| 2009 | Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial | Ferric carboxymaltose | 459 | 67 | |
| 2010 | Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) | Eplerenone | 2,737 | 69 | |
| Ivabradine and Outcomes in Chronic Heart Failure (SHIFT) study | Ivabradine | 6,558 | 60 | ||
| 2015 | Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial | Sacubitril and valsartan vs. enalapril | 8,399 | 64 | |
| Total: 84,747 | Mean age: 64 |
Older, frail patients have poorer organ function owing to age-related decline or chronic disease; this progressive loss of organ function can alter the pharmacokinetic and pharmacodynamic responses of a medicine. As people age, so do their physical attributes; lean body mass reduces, as does total body water volume, and adipose tissue is increased — all altering the expected pharmacokinetic process. Pharmacodynamic changes occur owing to changes in the expected action at receptor sites or the affinity for the receptor. Age-related changes make the older, frailer population more susceptible to drug adverse effects, especially those with chronic conditions[40]
.
Pharmacokinetics
Absorption can be reduced or slowed in older people but is generally not clinically significant.
Changes in body composition can significantly affect the distribution of a medicine and, subsequently, alter the intensity of its action. Water-soluble medicines taken by older people, many of whom have a reduced total body water volume, have a reduced volume of distribution resulting in higher blood concentrations for a specific dose. This often results in lower doses having an enhanced therapeutic effect. Lipid-soluble medicines, on the other hand, concentrate in fatty tissue. As people age, they tend to lose lean muscle mass, instead replacing this with adipose tissue. Consequently, lipid-soluble medicines have a wider area of distribution and clearance from fatty tissue can be slower. This, in turn, can increase half-life and cause more pronounced therapeutic effects. Therefore, in such cases, lower doses are generally recommended.
Liver metabolism is affected by a reduction in liver function. In older people, cytochrome P450 pathways are often diminished and drugs metabolised in this way may need adjusting. For example, many BBs are metabolised by cytochrome P450 pathways and most ACEIs are pro-drugs that require hepatic metabolism to convert them to their active form. The common thought for initiating evidence-based medical therapy in older people with heart failure is that a ‘start low, go slow and monitor regularly’ approach should be taken to prevent problems and adverse drug reactions[2],[41],[42]
.
Excretion is the most common and significant age-related pharmacokinetic change. Kidney function is easily monitored via simple serum creatinine and electrolyte testing, and the thresholds in UK and international guidance for reducing medicine doses are well established[2],[35],[43]
, meaning doses can be adjusted relatively easily.
A reduction in kidney blood flow and glomerular filtration rates, often owing to reduced perfusion, comorbid kidney disease and iatrogenic effects, can commonly lead to the accumulation and toxicity of many medicines and dose reductions are often required. This particularly applies to that of ACEIs and MRAs, which have kidney-dependent pharmacokinetics, exacerbating effects and increasing the risk of hyperkalaemia[44]
.
Pharmacodynamics
The concentration of the drug at the receptor, receptor response, pre-receptor events within cells and homoeostatic mechanisms can all result in altered pharmacodynamics[45]
. In older people, impaired homoeostatic mechanisms can increase susceptibility to adverse effects, such as confusion, urinary retention, urinary incontinence, constipation, hypothermia and postural hypotension.
A single serum concentration can produce both beneficial and harmful effects[46]
. The same plasma concentration of a drug may be associated with a higher risk of adverse effects in older patients compared with younger ones. Patient-specific factors, including age, gender, ethnicity, genetics and disease, may also affect pharmacodynamics[47]
. Pharmacodynamic changes associated with age are complex and require an individualised and holistic approach, and must be examined separately for each individual drug[48]
.
Sensitivity to beta-adrenergic agents declines with age; this is true for both agonists and antagonists acting on the cardiac receptors[49]
. Owing to alterations in the signal transduction pathway, increasing doses of BBs might be necessary to provide the same response, leading to a greater risk of adverse events[50]
.
There can be reduced diuretic response owing to impaired tubular secretions and higher doses are often required to achieve a therapeutic response[44]
. However, dysregulation of the baroreceptor reflex in older patients can cause increased sensitivities owing to volume depletion induced by diuretics[48]
. This leads to a potential increased risk of postural hypotension and falls. Older patients are more at risk of developing electrolyte disturbance when using diuretics; particularly more than one type of diuretic[49]
.
ACEIs do not show age-related pharmacodynamic changes in older patients[48]
; however, as previously discussed, their effects can be changed by metabolism impairment.
Age-related changes have resulted in recommendations to start with smaller initial doses than in younger adults and the dose should then be titrated to a clearly defined clinical response[2],[41],[42],[49]
.
Medicine optimisation
In addition to age-related changes in pharmacokinetics and pharmacodynamics, older people can often be affected by polypharmacy and multimorbidities that make them much more susceptible to adverse drug effects[49]
. Polypharmacy is described as taking ‘five or more medicines’[50]
. Inappropriate polypharmacy is of particular concern in older people owing to associated negative health outcomes, including adverse drug reactions, poor adherence and geriatric syndromes such as urinary incontinence, cognitive impairment and impaired balance leading to falls[51],[52]
. Evidence suggests that polypharmacy is associated with increased risks of unplanned hospital admission, mortality and admission to care homes[53]
.
In heart failure management, the treatment aim is to titrate medicines to maximum tolerated doses while aiming for the target dose for a particular medicine[2],[35]
. This becomes difficult in an older, frail population as we are adding multiple medicines to an often existing polypharmacy regimen. To ensure patients receive the most effective heart failure treatment, a review of existing medicines with potential deprescribing may be necessary.
Structured medicine review is recommended for all adults with chronic and long-term conditions and those taking multiple medicines[54]
. A review should include all medicines taken by the patient, with a view to continued appropriateness. Many tools have been developed to aid the decision-making process around appropriateness of medicines, including STOPP/START[55]
and the Beers Criteria[56]
. The STOPPFrail criteria have been specifically designed to look at the appropriateness of medicines in frail adults and advocates reviewing older patients who meet all of the following criteria: end-stage irreversible pathology, poor one-year survival prognosis, severe functional impairment or severe cognitive impairment (or both), and symptom control being the priority rather than prevention of disease progression[57]
.
In older, frail patients, particular attention should be given to medicines with anticholinergic effects that are associated with an increased risk of cognitive impairment, all-cause mortality and falls[58]
. Anticholinergic use has been linked with worsening cognitive performance either with an acute (delirium) or chronic (mild cognitive impairment) impact[59]
. Recent research also indicates a possible link between mortality and the number and potency of anticholinergic agents prescribed[60]
.
Co-prescribed medicines without an immediate effect on symptom relief or quality of life[35]
, as well as other medicines that could adversely affect a patient’s heart failure status, should be reviewed and discontinued where possible. Reviewing the continued need for calcium channel blockers and nitrates may be useful in allowing up-titration of evidence-based heart failure therapy. Lipid-lowering drugs are only beneficial over the long term and future prognosis should be considered. Diuretics should be used at the lowest effective dose and, in general, heart failure medicines should be titrated in small increments, with close monitoring undertaken to prevent adverse drug reactions.
Non-cardiac medicines should also be reviewed for continued appropriateness to reduce the polypharmacy burden (i.e. drugs for urgency and frequency; is it the diuretics causing the issue? Is the patient now catheterised and, if so, do they still require medicines such as finasteride, oxybutynin, solifenacin or alpha blockers?). There is no benefit of such treatment where a long-term catheter is in place and the risk of adverse drug reactions is increased. Antidiabetic medicines should be reviewed as stringent glycaemic control is unnecessary in frail older patients. It is, however, important to take a patient-centred approach to such decisions, including the patient in the decision-making process where possible, not a ‘one size fits all’ approach.
It is important to ensure patients are prescribed evidence-based heart failure treatment regardless of age, owing to the extrapolated evidence base. However, it is also important to ensure considerations are given to the treatment complexities of older people as their response may well be different than expected. A holistic view of patient care in older, frail people with heart failure may be more beneficial than the standard target-driven approach commonly applied to younger people with heart failure.
Devices and surgery
Many older patients will also meet the criteria for complex devices or surgical intervention and consideration should be given to these options. This should include thorough discussion with the patient before determining an agreed approach to care. Cardiac resynchronisation therapy can have symptomatic benefit in older patients besides reducing morbidity, cardiac events and hospitalisations, including quality of life, cognitive and functional status[61]
. Conversely, there is also an increased risk of complications following such procedures in older patients[62],[63]
. Implantable cardioverter defibrillators, used to prevent sudden cardiac death related to dangerous arrhythmias, are likely to become less appropriate in many older patients because the decision to implant them takes into account one-year life expectancy, that they have no impact on clinical symptoms or quality of life, and the evidence base to support their use in this population is lacking[64]
. However, they should be discussed with the patient, with a detailed explanation of benefits and risks, as well as information given on the deactivation of such devices, so as to prevent difficult conversations later down the line.
The complications associated with major cardiac surgery become more prevalent in older patients[65]
and assessment of the appropriateness of such interventions should be made by specialist teams, although it is worth noting that patients often decline referral for consideration of surgery.
Holistic care planning
The comprehensive geriatric assessment (CGA) is the gold standard for management of frailty in older people (see Figure 2)[18]
. It involves a holistic, multidimensional, interdisciplinary assessment of an individual by several specialists of many disciplines in older people’s health and has been proven to be associated with improved outcomes in a variety of settings[20]
. Frail older people receiving inpatient CGA are more likely to return home, are less likely to experience cognitive or functional decline and have lower in-hospital mortality[17]
. In a heart failure population, a poor score on a CGA has been linked with worse prognoses for one-month mortality[66]
, one-year mortality in patients hospitalised with heart failure[67]
, and two-year mortality in older patients with heart failure[68]
. Despite the evident link to prognoses, the application of the CGA as an interventional process to improve outcomes has not been established in a heart failure population. It may be a useful tool for frail people with heart failure but requires further research to confirm.
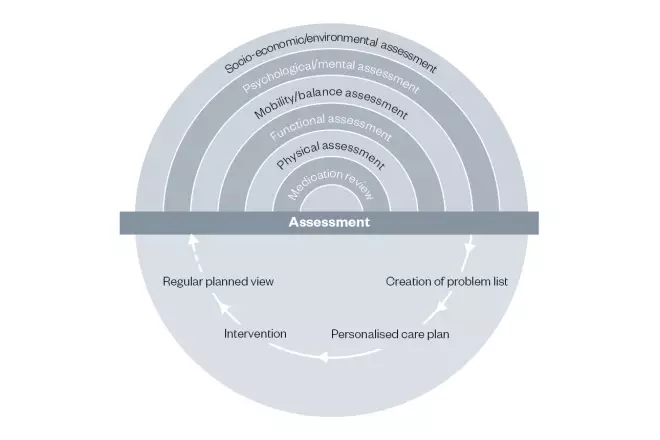
Figure 2: Comprehensive geriatric assessment
Source: British Geriatrics Society. Comprehensive geriatric assessment toolkit for primary care practitioners. Available at: https://www.bgs.org.uk/sites/default/files/content/resources/files/2019-02-08/BGS%20Toolkit%20-%20FINAL%20FOR%20WEB_0.pdf (accessed July 2019)
A multidimensional assessment of the patient is required in order to create a specific care plan for them, including arrangement of interventions to support the plan
Palliation
Palliative care was initially developed to provide holistic and timely symptom-based care for patients with non-curable cancer, but this should also be available and offered to patients with non-malignant, life-limiting diseases, such as heart failure and frailty[69]
. It is now recognised that the terminal phase of heart failure may be comparable to cancer, both in terms of symptoms and distress[70]
. A previous study showed that only 4% of people with heart failure receive palliative care input[71]
. When people with heart failure remain symptomatic despite maximal treatment, or frailty prevents further intervention, appropriate end-of-life care should focus on maintaining quality of life[69]
. Therefore, patients should be given the opportunity for advanced care planning. Advanced care planning is difficult in heart failure because of its unpredictable disease trajectory. However, with the diagnosis of frailty in older patients being associated with worse outcomes, it would make advanced care planning a sensible approach. The main aims of advanced care planning are to establish an individualised plan based on holistic assessment, appropriate clinical treatment and the patient’s personal wishes[72]
. Advanced care plans can include information on resuscitation status and treatment plans in the event of certain circumstances (e.g. falls or infections), and can indicate the appropriate pathways to be followed in line with the patient’s wishes. This improves end-of-life care, patient and family satisfaction, and reduces stress, anxiety and depression in surviving relatives[72]
.
Summary
Heart failure and frailty are complex interwoven conditions that are both linked with worse outcomes independently and especially in combination. The diagnosis of either condition can often be masked by other long-term conditions and the delivery of treatment goals can be problematic. Nevertheless, older, frail patients should be offered evidenced-based therapies to treat heart failure in the same way as younger patients with additional considerations of increased risk of adverse reactions to medical therapy. Considerations should also be given to the holistic management of patients, CGAs and relevant future care planning with the patient and/or carers as appropriate.
References
[1] British Heart Foundation. CVD Statistics. 2014. Available at: https://www.bhf.org.uk/informationsupport/publications/statistics/cardiovascular-disease-statistics-2014 (accessed July 2019)
[2] National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management. NICE guideline [NG106]. 2018. Available at: https://www.nice.org.uk/guidance/ng106 (accessed July 2019)
[3] National Institute for Cardiovascular Outcomes Research. National Heart Failure Audit 2016–2017. 2016. Available at: https://www.nicor.org.uk/national-cardiac-audit-programme/heart-failure-heart-failure-audit/ (accessed July 2019)
[4] National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. NICE clinical guideline [CG187]. 2014. Available at: https://www.nice.org.uk/guidance/cg187 (accessed July 2019)
[5] Denfeld QE, Winters-Stone K, Mudd JO et al. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 2017;236:283–289. doi: 10.1016/j.ijcard.2017.01.153
[6] Jha SR, Ha HS, Hickman LD et al. Frailty in advanced heart failure: a systematic review. Heart Fail Rev 2015;20(5):553–560. doi: 10.1007/s10741-015-9493-8
[7] Vidán MT, Blaya-Novakova V, Sánchez E et al. Prevalence and prognostic impact of frailty and it components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016;18(7):869–875. doi: 10.1002/ejhf.518
[8] MartÃn-Sánchez FJ, RodrÃguez-Adrada E, Vidan MT et al. Representing the members of the OAK Register Investigators. Impact of frailty and disability on 30-day mortality in older patients with acute heart failure. Am J Cardiol 2017;120(7):1151–1157. doi: 10.1016/j.amjcard.2017.06.059
[9] Cacciatore F, Abete P, Mazzella F et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005;35(12):723–730. doi: 10.1111/j.1365-2362.2005.01572.x
[10] MartÃn-Sánchez FJ, RodrÃguez-Adrada E, Mueller C et al. The effect of frailty on 30-day mortality risk in older patients with acute heart failure attended in the emergency department. Acad Emerg Med 2017;24(3):298–307. doi: 10.1111/acem.13124
[11] RodrÃguez-Pascual C, Paredes-Galán E, Ferrero-MartÃnez AI et al. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol 2017;236:296–303. doi: 10.1016/j.ijcard.2017.02.016
[12] Madan SA, Fida N, Barman P et al. Frailty assessment in advanced heart failure. J Card Fail 2016;22(10):840–844. doi: 10.1016/j.cardfail.2016.02.003
[13] McNallan SM, Singh M, Chamberlain AM et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail 2013;1(2):135–141. doi: 10.1016/j.jchf.2013.01.002
[14] Butts B & Gary R. Coexisting frailty, cognitive impairment, and heart failure: implications for clinical care. J Clin Outcomes Manag 2015;22(1):38–46. PMID: 26594103
[15] Buck HG & Riegel B. The impact of frailty on health related quality of life in heart failure. Eur J Cardiovasc Nurs 2011;10(3):159–166. doi: 10.1016/j.ejcnurse.2010.06.001
[16] Denfeld QE, Winters-Stone K, Mudd JO et al. Identifying a relationship between physical frailty and heart failure symptoms. J Cardiovasc Nurs 2018;33(1):E1–E7. doi: 10.1097/JCN.0000000000000408
[17] Ellis G & Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2005;71(1):45–59. doi: 10.1093/bmb/ldh033
[18] British Geriatrics Society. Fit for frailty. Consensus best practice guidance for the care of older people living with frailty in community and outpatient settings. 2014. Available at: https://www.bgs.org.uk/sites/default/files/content/resources/files/2018-05-14/fff2_short_0.pdf (accessed July 2019).
[19] Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence of for a phenotype. J Gerontology 2001;56(3):146–156. doi: 10.1093/gerona/56.3.M146
[20] Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173(5):489–495. doi: 10.1503/cmaj.050051
[21] Rolfson DB, Majumdar SR, Tsuyuki RT et al. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006;3595:526–529. doi: 10.1093/ageing/afl041
[22] Steverink N, Slaets JPJ, Schuurmans H & van Lis M. Measuring frailty: developing and testing the GFI (Groningen frailty indicator). Gerontologist 2001;41(1):236–237
[23] McDonagh J, Martin L, Ferguson C, Jha SR et al.. Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs 2017;17(1):23–35. doi: 10.1177/1474515117708888
[24] Gale CR, Cooper C & Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing 2015;44(1):162–165. doi: 10.1093/ageing/afu148
[25] Age UK. Briefing: Health and care of older people in England 2017. 2017. Available at: https://www.ageuk.org.uk/Documents/EN-GB/For-professionals/Research/The_Health_and_Care_of_Older_People_in_England_2016.pdf?dtrk=true (accessed July 2019).
[26] UK Department of Health and Social Care. Discharging older patients from hospital. 2016. London: National Audit Office. Available at: https://www.nao.org.uk/wp-content/uploads/2015/12/Discharging-older-patients-from-hospital.pdf (accessed July 2019)
[27] NHS England. Safe, compassionate care for frail older people using an integrated care pathway: Practical guidance for commissioners, providers and nursing, medical and allied health professional leaders. 2014. Available at: https://www.england.nhs.uk/wp-content/uploads/2014/02/safe-comp-care.pdf (accessed July 2019)
[28] Age UK. Briefing: the health and care of older older people in England. 2015. Available at: http://www.cpa.org.uk/cpa/docs/AgeUK-Briefing-TheHealthandCareofOlderPeopleinEngland-2015.pdf (accessed July 2019)
[29] Murad K, Goff DC, Morgan TM et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality. JACC Heart Fail 2015;3(7):542–550. doi: 10.1016/j.jchf.2015.03.004
[30] Gastelurrutia P, Benrimoj SI, Espejo J et al. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail 2011;17(3):217–223. doi: 10.1016/j.cardfail.2010.10.009
[31] Salive ME. Multimorbidity in older adults. Epidemiol Rev 2013;35(1):75–83. doi: 10.1093/epirev/mxs009
[32] Palladino R, Tayu Lee J, Ashworth M et al. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 european countries. Age Ageing 2016;45:431–435. doi: 10.1093/ageing/afw044
[33] Morley JE. Inappropriate drug prescribing and polypharmacy are major causes of poor outcomes in long-term care. J Am Med Dir Assoc 2014;15(11):780–782. doi: 10.1016/j.jamda.2014.09.003
[34] Health Education England. Older People. CPPE. 2015. Available at: https://www.cppe.ac.uk/wizard/files/tasters/older-p-03_taster.pdf (accessed July 2019).
[35] Ponikowski P, Voors AA, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128
[36] Ray P, Delerme S, Jourdain P & Chenevier-Gobeaux C. Differential diagnosis of acute dyspnea: the value of B natriuretic peptides in the emergency department. QJM 2008;101(11):831–843. doi: 10.1093/qjmed/hcn080
[37] Tsai SH, Lin YY, Chu SJ et al. Interpretation and use of natriuretic peptides in non-congestive heart failure settings. Yonsei Med J 2010;51(2):151–163. doi: 10.3349/ymj.2010.51.2.151
[38] Van Riet EE, Hoes AW, Wagenaar KP et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18(3):242–252. doi: 10.1002/ejhf.483
[39] Office for National Statistics. National life tables, UK: 2013 to 2015. 2017. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/20132015 (accessed July 2019)
[40] Hämmerlein A, Derendorf H & Lowenthal DT. Pharmacokinetic and pharmacodynamic changes in the elderly. Clin Pharmacokinet 1998;35(1):49–64. doi: 10.2165/00003088-199835010-00004
[41] Samala RD, Navas V, Saluke E & Ciocon JO. Heart failure in frail, older patients: we can do ‘MORE’. Cleve Clin J Med 2011;8(12):838–845. doi: 10.3949/ccjm.78a.11085
[42] Walker DM, Gale CP, Lip G et al. Frailty and the management of patients with acute cardiovascular disease: a position paper from the Acute Cardiovascular Care Association. Eur Heart J 2018;7(2):176–193. doi: 10.1177/2048872618758931
[43] Dunleavy A & Ashley C. The Renal Drug Handbook: The Ultimate Prescribing Guide for Renal Practitioners. 5th ed. CRC Press: Boca Raton; 2018
[44] Hartmann B, Czock D & Keller F. Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int 2010;107(37):647–656. doi: 10.3238/arztebl.2010.0647
[45] Midlov P. Pharmacokinetic and pharmacodynamics in the elderly. OA Elderly Medicine 2013;01:1(1):1. doi: 10.13172/2054-734x-1-621
[46] Aymans C, Keller F, Maus S et al. Review on pharmacokinetics and pharmacodynmics and the ageing kidney. Clin J Am Soc Nephrol 2010;5:314–327. doi: 10.2215/CJN.03960609
[47] Bowie MW & Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother 2007;5:263–303. doi: 10.1016/j.amjopharm.2007.10.001
[48] Corsonello A, Pedone C & Antonelli Incalzi R. Age related pharmacokinetics and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem 2010;17:571–584. doi: 10.2174/092986710790416326
[49] Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 2003;843–853. PMID: 12915206
[50] Fulton MM & Riley AE. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract 2005;17:123–132. PMID: 15819637
[51] The King’s Fund. Polypharmacy and medicines optimisation: making it safe and sound. 2013. Available at: https://www.kingsfund.org.uk/publications/polypharmacy-and-medicines-optimisation (accessed July 2019)
[52] Patterson SM, Hughes C, Kerse N et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2012(5):CD008165. doi: 10.1002/14651858.CD008165.pub2
[53] Yorkshire and Humber AHSN Improvement Academy. Effectiveness matters: reducing harm from polypharmacy in older people. 2017. Available at: https://www.york.ac.uk/media/crd/effectiveness-matters-aug-2017-polypharmacy.pdf (accessed July 2019)
[54] National Institute for Health and Care Excellence. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. NICE guideline [NG5]. 2015. Available at: https://www.nice.org.uk/guidance/ng5 (accessed July 2019)
[55] Gallagher P, Ryan C, Byrne S et al. STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther 2008;46(2):72–78. doi: 10.5414/cpp46072
[56] Fick DM, Semla TP, Beizer J et al.; American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227–2246. doi: 10.1111/jgs.13702
[57] Lavan AH, Gallagher P, Parsons C & O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing 2017;46(4):600–607. doi: 10.1093/ageing/afx005
[58] National Institute for Health and Care Excellence. Multimorbidity and polypharmacy. NICE key therapeutic topic [KTT18]. 2017. Available at: https://www.nice.org.uk/advice/ktt18 (accessed July 2019)
[59] Campbell N, Boustani M, Limbil T et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging 2009;4:225–233. PMID: 19554093
[60] PrescQIPP. Bulletin 140: Anticholinergic drugs. 2016. Available at: https://www.prescqipp.info/our-resources/bulletins/bulletin-140-anticholinergic-drugs/ (accessed July 2019)
[61] Bontempi L, Vassanelli F & Curnis A. Resynchronization therapy in the elderly. Eur J Arrhythm Electrophysiol 2016;2(1):18–19. doi: 10.17925/EJAE.2016.02.01.18
[62] Mikhaylov EN & Lebedev DS. Cardiac resynchronization in the elderly is beneficial, but could we implant our devices in old patients safer? J Geriatr Cardiol 2016;13(3):277–278. doi: 10.11909/j.issn.1671-5411.2016.03.014
[63] Suleiman M, Goldenberg I, Haim M et al. Clinical characteristics and outcomes of elderly patients treated with an implantable cardioverter-defibrillator or cardiac resynchronization therapy in a real-world setting: data from the Israeli ICD Registry. Heart Rhythm 2014;11(3):435–441. doi: 10.1016/j.hrthm.2013.12.003
[64] Brignole M, Auricchio A, Baron-Esquivias G et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150
[65] Lee DH, Buth KJ, Martin B et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437
[66] Pilotto A, Addante F, Franceschi M et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail 2010;3(1):14–20. doi: 10.1161/CIRCHEARTFAILURE.109.865022
[67] Sánchez E, Vidán MT, Serra JA et al. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart 2011;97(19):1602–1606. doi: 10.1136/hrt.2011.227504
[68] Rodriguez-Pascual C, Paredes-Galan E, Vilches-Moraga A et al. Comprehensive geriatric assessment and 2-year mortality in elderly patients hospitalized for heart failure. Circ Cardiovasc Qual Outcomes 2014;7:251–258. doi: 10.1161/CIRCOUTCOMES.113.000551
[69] Koller K & Rockwood K. Frailty in older adults: implications for end-of-life care. Cleve Clin J Med 2013;80:168–174. doi: 10.3949/ccjm.80a.12100
[70] Hockley JM, Dunlop R & Davies RJ. Survey of distressing symptoms in dying patients and their families in hospital and the response to a symptom control team. BMJ 1988;296:1715–1717. doi: 10.1136/bmj.296.6638.1715
[71] Gibbs JSR, McCoy ASM, Gibbs LME et al. Living with and dying from heart failure: the role of palliative care.Heart 2002;88:ii36–ii39. doi: 10.1136/heart.88.suppl_2.ii36
[72] Detering KM, Hancock AD, Reade MC & Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. doi: 10.1136/bmj.c1345