Panther Media GmbH / Alamy Stock Photo
Hepatitis C virus (HCV) is a blood-borne, positive-stranded RNA virus within the genus Hepacivirus and family Flaviviridae
[1]
. Chronic infection causes liver cirrhosis and liver cancer and, alongside hepatitis B, it is a leading cause of mortality worldwide[2]
. The existence of HCV was first suspected in blood transfusion recipients in the United States who developed post-transfusion hepatitis despite testing negative for hepatitis A and B, and was finally isolated in 1989. Soon after its discovery, an assay was developed to detect the presence of HCV antibodies, which facilitated the screening of donated blood products by the early 1990s, further research into the epidemiology of the virus and the development of curative treatments[3]
[4]
[5]
.
What symptoms does it cause?
Most people with acute HCV (70–80%) do not develop symptoms, however, in a minority of cases, individuals may experience nausea, dark urine, anorexia, abdominal pain and jaundice[6]
. Only a minority of cases (15–40%) spontaneously clear the acute infection, with women, younger persons and those with favourable genetic polymorphisms being less likely to develop chronic disease[6]
[7]
[8]
[9]
.
Chronic HCV can be asymptomatic but may be characterised by a range of non-specific symptoms including sweats, rashes and mood disturbances which, while often considered mild and non-specific, are associated with a reduced quality of life[10]
. Due to the non-specific nature of the symptoms, many people with chronic infection do not present to health services and, of those that do, few undergo testing for HCV.
What are the consequences of chronic infection?
Over the course of 20 years since initial infection, around 20% of persons with chronic HCV develop severe scarring of the liver (cirrhosis), but this process can be accelerated in persons who consume excessive quantities of alcohol or who are co-infected with HIV[11]
[12]
. While cirrhosis itself may be asymptomatic, it can lead to decompensated liver disease, which is characterised by jaundice, bleeding and fluid within the abdomen (ascites), and primary cancer of the liver, known as hepatocellular carcinoma (HCC).
Having HCV can reduce life expectancy by 8–12 years[13]
. People with compensated cirrhosis (where there is liver scarring but the organ is essentially functioning normally) have a prognosis of around 12 years, but if they have decompensated disease median survival is reduced to just 2 years[14]
.
Who is at risk?
Chronic HCV is estimated to affect 2–3% of the world population, although the disease burden varies in different regions[15]
. The highest disease prevalence is in Egypt, where HCV was probably originally transmitted via a national vaccination programme in the 1950s and is now primarily sustained at endemic levels through contaminated medical and dental equipment[15]
[16]
. In other areas, including the UK, the prevalence is lower and since screening of donated blood products has been introduced, injecting drug use with non-sterile equipment is the primary vector for transmission[15]
.
However, other potential routes of transmission persist and include receipt of blood products (before 1991), receiving healthcare abroad with non-sterile equipment, sex with an infected partner, transmission from mother to baby (so-called vertical transmission), non-sterile tattoo equipment and haemodialysis[17]
.
The changing treatment landscape
The treatment of HCV has changed dramatically over the last few decades[18]
. In the late 1980s, interferon-α (IFN-α) was used to treat patients with ‘non-A non-B’ hepatitis (before HCV was discovered) and, although this was effective in some cases, many patients did not respond or later relapsed. Ribavirin was added to a longer acting IFN-α preparation (pegylated [PEG]-interferon) and the levels of sustained virological response (SVR) increased significantly[19]
. This became the mainstay of treatment, but many patients particularly with genotype 1 HCV still did not achieve a SVR to therapy, and treatment was associated with numerous side effects[20]
. Consequently, many patients (10–20%) withdraw from therapy and others (20–30%) needed dose modification during treatment[20]
. Furthermore, treatment had numerous contraindications, including decompensated liver disease (meaning those who were severely affected by HCV could not receive treatment) and pre-existing severe psychiatric illnesses.
From 2011, a new class of directly acting anti-viral drugs (DAAs), called protease inhibitors, were developed and given in combination with PEG-interferon and ribavirin (as so-called ‘triple therapy’) to patients with genotype 1 disease. This improved the proportion of patients achieving SVR but continued to be associated with side effects, contraindications to therapy and drug–drug interactions[21],[22]
. This has led to the development of other classes of DAAs including NS3/4A inhibitors, NS5A inhibitors and NS5B inhibitors. Given in combination, these drugs are more than 90% effective at achieving SVR, and, because they can be given without combination with PEG-interferon and ribavirin, have few side effects and few contraindications[23],[24]
.
Hepatitis C (HCV) elimination by 2030
The dramatic progress in drug development for HCV has resulted in the World Health Organization (WHO) setting a target for HCV elimination by 2030. However, the WHO also highlighted that a key part of achieving this goal was to reduce the number of undiagnosed HCV infections and increase the number of people engaged with treatment[25]
.
Most infections in the UK are in people who inject drugs (PWID); up to 50% of these individuals are not aware they are infected and many of those who are aware, have not been engaged with treatment services[26]
. This is important because modelling studies have highlighted that treating PWID can actually prevent further infections and lead to a faster reduction in the overall population prevalence of HCV[27]
[28]
. Therefore, it follows that to achieve the WHO target, identifying HCV in PWID and treating these cases is a priority.
Targeted screening and treatment for HCV — a role for community pharmacy
There are numerous barriers to testing and treatment in PWID that need to be overcome[29],[30],[31],[32]
. Attempts have been made to address these; in the UK a series of national action plans and National Institute for Health and Care Excellence (NICE) guidance have urged action to increase HCV testing, and this has prompted initiatives including GP record screening for people at risk of HCV, screening in emergency departments, screening in prisons and widespread testing in drug support centres[33],[34]
[35],[36],[37],[38]
.
There is also growing evidence of the potential effectiveness of HCV testing in community pharmacies. On the Isle of Wight, Hampshire, pharmacy screening was effective at engaging individuals with a range of risk factors for HCV using dry-blood spot testing and highlighted the importance of a robust referral pathway for engaging positive cases with treatment. It also demonstrated the importance of the unique relationships between community pharmacists and PWID attending for needle exchange or opiate substitution therapy in identifying and counselling PWID to undertake a test[39]
.
In Dundee, HCV testing has also been provided in community pharmacy but this has been extended to include pharmacy service provision of treatment[20]
. This has included a feasibility cluster randomised trial, which indicated that PWID were significantly more likely to engage with treatment if they were managed through their community pharmacy[40]
.
What would a pharmacy-based model of care look like in the future?
With the change in the treatment landscape for HCV, community pharmacists are uniquely placed, in having daily contact with PWID, to deliver HCV testing and treatment to this at-risk population. However, there are potential pitfalls in this approach that need to be considered when designing a future service (see Box 1).
Box 1: Benefits and barriers to community pharmacy-delivered care for hepatitis C virus (HCV)
Potential benefits of community pharmacy testing and treatment for HCV:
- Increased engagement in testing and treatment of people who inject drugs (PWID);
- Improved concordance with antiviral therapy, particularly among PWID;
- Reduced costs to local hospital services;
- Increased total capacity for treatment provision;
- Greater convenience for patients.
Potential barriers to community pharmacy testing and treatment for HCV:
- Limited drug availability;
- High community pharmacy staff turnover;
- Costs of community pharmacists’ time and testing equipment;
- Community pharmacy treatment room availability;
- Lack of HCV specialist nurse time/capacity to visit pharmacy;
- Lack of portable transient elastography equipment.
The study on the Isle of Wight was preceded by a national pilot of pharmacy screening in 2010, which identified more than 10 cases of HCV on the Isle of Wight, but only one patient attended a secondary care appointment and none received treatment. The reasons for this are unclear but at the time there was no locally available treatment and the referral pathway for positive cases was complex. Additionally, the later study noted that a small number of pharmacies provided the majority of testing[39]
[41]
. These pharmacies were characterised by highly motivated long-term staff and, importantly, also had a treatment room to conduct testing in a private environment.
To make a community pharmacy a ‘one-stop shop’ for HCV care, where a positive test leads to on-site investigations and treatment, further challenges are likely to be encountered. These include practicalities such as the availability of a bed to conduct transient elastography (currently the recommended assessment modality for liver fibrosis), the necessary training for community pharmacists, and the close integration between the clinical HCV operational delivery network and community services. A potentially more difficult hurdle to overcome will be establishing where funding for such community pharmacy services will come from in the fragmented commissioning environment[42]
[43]
. Figure 1 summarises how a ‘one-stop shop’ community pharmacy service could be structured.
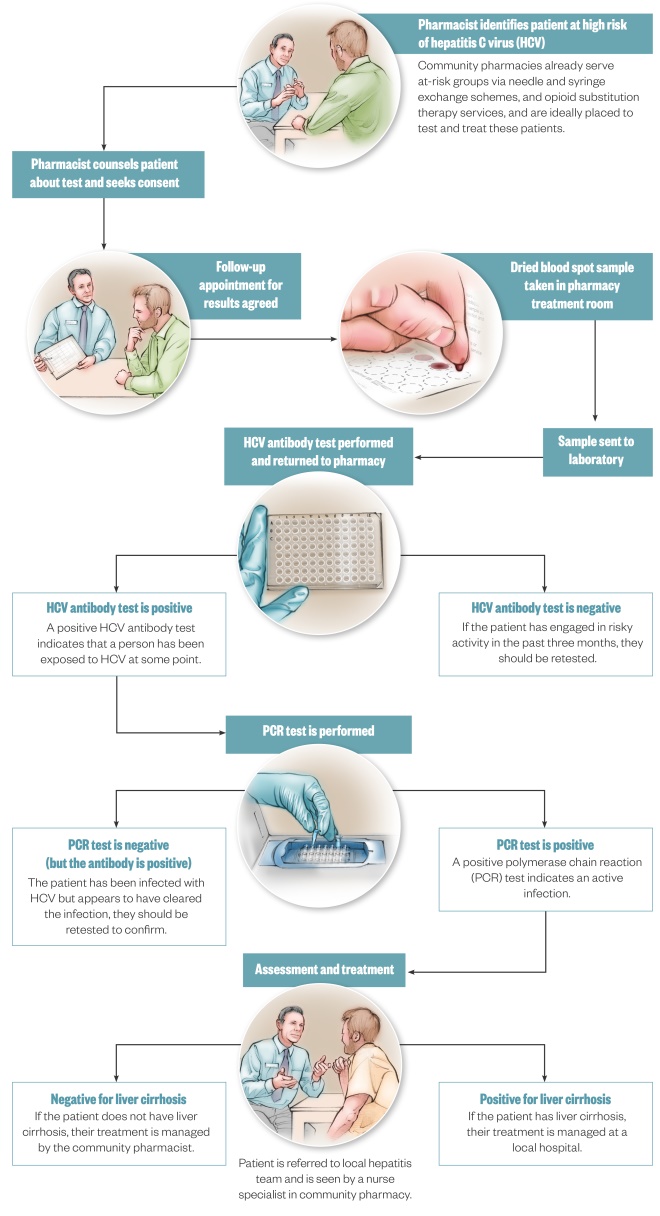
Figure 1: A potential care model for hepatitis C virus (HCV) in community pharmacy
Illustrations by Juliet Percival
Chronic hepatitis C virus (HCV) is estimated to affect 2–3% of the world population and numerous barriers exist to testing and treatment, especially in people who inject drugs (PWID). In the UK, a series of national action plans and National Institute for Health and Care Excellence (NICE) guidance have urged action to increase HCV testing, and this has prompted initiatives including GP record screening for people at risk of HCV, screening in emergency departments, screening in prisons and widespread testing in drug support centres. There is also growing evidence of the potential effectiveness of HCV testing in community pharmacies.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] McGarvey MJ & Houghton M. Structure and molecular virology. In: Thomas HC, Lok AS, Locarnini S, Zuckerman A, eds. Viral Hepatitis. 4th Ed. Chichester: Wiley-Blackwell; 2014:221–235.
[2] Stanaway JD, Flaxman AD, Naghavi M et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;0(0):988–997. doi: 10.1016/S0140-6736(16)30579-7
[3] Alter HJ, Purcell RH, Shih JW et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med 1989;321(22):1494–1500. doi: 10.1056/NEJM198911303212202
[4] Choo QL, Kuo G, Weiner AJ et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989;244(4902):359–362. doi: 10.1126/science.2523562
[5] Ward JW. Hepatitis C virus: the 25-year journey from discovery to cure. Hepatology 2014;60(5):1479–1482. doi: 10.1002/hep.27377
[6] Westbrook RH & Dusheiko G. Natural history of hepatitis C. J Hepatol 2014;61(1):S58–S68. doi: 10.1016/j.jhep.2014.07.012
[7] Grebely J, Raffa JD, Lai C et al. Factors associated with spontaneous clearance of hepatitis C virus among illicit drug users. Can J Gastroenterol 2007;21(7):447–451. doi: 10.1155/2007/796325
[8] Bulteel N, Partha Sarathy P, Forrest E et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J Hepatol 2016;65(2):266–272. doi: 10.1016/j.jhep.2016.04.030
[9] Ge D, Fellay J, Thompson AJ et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461(7262):399–401. doi: 10.1038/nature08309
[10] Foster GR, Goldin RD & Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998;27(1):209–212. doi: 10.1002/hep.510270132
[11] Hassan M, Hwang L, Hatten CJ et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36(5):1206–1213. doi: 10.1053/jhep.2002.36780
[12] Kramer JR, Giordano TP, Souchek J et al. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol 2005;100(1):56–63. doi: 10.1111/j.1572-0241.2005.40670.x
[13] Ryder SD. Outcome of hepatitis C infection: bleak or benign? J Hepatol 2007;47(1):4–6. doi: 10.1016/j.jhep.2007.04.006
[14] D’amico G, Garcia-Tsao G & Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013
[15] Ansaldi F, Orsi A, Sticchi L et al. Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol 2014;20(29):9633. doi: 10.3748/wjg.v20.i29.9633
[16] Pybus OG. The epidemiology and iatrogenic transmission of hepatitis C virus in Egypt: a bayesian coalescent approach. Mol Biol Evol 2003;20(3):381–387. doi: 10.1093/molbev/msg043
[17] Shepard CW, Finelli L & Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4
[18] Sarpel D, Carter D, Wasserman I et al. An update on the management of chronic hepatitis B and C infection. Clin Pharm 2017;9(8):247–256. doi: 10.1211/CP.2017.20203306
[19] Di Bisceglie AM, Martin P, Kassianides C et al. Recombinant interferon alfa therapy for chronic hepatitis C. N Engl J Med 1989;321(22):1506–1510. doi: 10.1056/NEJM198911303212204
[20] Manns MP, Wedemeyer H & Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55(9):1350–1359. doi: 10.1136/gut.2005.076646
[21] Sarrazin C, Rouzier R, Wagner F et al. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon α-2b for genotype 1 nonresponders. Gastroenterology 2007;132(4):1270–1278. doi: 10.1053/j.gastro.2007.01.041
[22] Price JC, Murphy RC, Shvachko VA et al. Effectiveness of telaprevir and boceprevir triple therapy for patients with hepatitis C virus infection in a large integrated care setting. Dig Dis Sci 2014;59(12):3043–3052. doi: 10.1007/s10620-014-3294-0
[23] Foster GR, Afdhal N, Roberts SK et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612
[24] Afdhal N, Zeuzem S, Kwo P et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454
[25] World Health Organization (WHO). Combating hepatitis B and C to reach elimination by 2030. 2016. Available at: http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ (accessed November 2017)
[26] Public Health England. Hepatitis C in the UK. 2016. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/538321/hpr2316_uampwid.pdf (accessed November 2017)
[27] Martin NK, Vickerman P, Foster GR et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol 2011;54(6):1137–1144. doi: 10.1016/j.jhep.2010.08.029
[28] Innes H, Goldberg D, Dillon J et al. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut 2015;64(11):1800–1809. doi: 10.1136/gutjnl-2014-308166
[29] Richmond JA, Dunning TL & Desmond PV. Health professionals’ attitudes toward caring for people with hepatitis C. J Viral Hepat 2007;14(9):624–632. doi: 10.1111/j.1365-2893.2007.00849.x
[30] Parkes J, Roderick P, Bennett-Lloyd B et al. Variation in hepatitis C services may lead to inequity of heath-care provision: a survey of the organisation and delivery of services in the United Kingdom. BMC Public Health 2006;6(1):3. doi: 10.1186/1471-2458-6-3
[31] World Health Organization (WHO). Barriers and facilitators to hepatitis C treatment for people who inject drugs. A qualitative study. Available at: http://www.euro.who.int/en/health-topics/communicable-diseases/hivaids/publications/2012/barriers-and-facilitators-to-hepatitis-c-treatment-for-people-who-inject-drugs.-a-qualitative-study (accessed November 2017)
[32] Harris M & Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013;10:7. doi: 10.1186/1477-7517-10-7
[33] National Institute for Health and Care Excellence (NICE). Hepatitis B and C: ways to promote and offer testing to people at increased risk of infection. Public Health Guideline [PH43]. Available at: http://www.nice.org.uk/guidance/ph43 (accessed November 2017)
[34] Department of Health. Hepatitis C Action Plan for England. Available at: http://www.nhs.uk/hepatitisc/SiteCollectionDocuments/pdf/hepatitis-c-action-plan-for-england.pdf (accessed November 2017)
[35] Cullen BL, Hutchinson SJ, Cameron SO et al. Identifying former injecting drug users infected with hepatitis C: an evaluation of a general practice-based case-finding intervention. J Public Health (Oxf) 2012;34(1):14–23. doi: 10.1093/pubmed/fdr097
[36] Galbraith JW, Franco RA, Donnelly JP et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology 2015;61(3):776–782. doi: 10.1002/hep.27410
[37] Merchant RC, Baird JR, Liu T et al. Brief intervention to increase emergency department uptake of combined rapid human immunodeficiency virus and hepatitis C screening among a drug misusing population. Acad Emerg Med 2014;21(7):752–767. doi: 10.1111/acem.12419
[38] Rumble C, Pevalin DJ & O’Moore É. Routine testing for blood-borne viruses in prisons: a systematic review. Eur J Public Health 2015;25(6):1078–1088. doi: 10.1093/eurpub/ckv133
[39] Buchanan R, Hassan-Hicks P, Noble K et al. Integrating community pharmacy testing for hepatitis C with specialist care. Clin Pharm 2016;8(8). doi: 10.1211/CP.2016.20201549
[40] Radley A, Tait J & Dillon JF. DOT-C: a cluster randomised feasibility trial evaluating directly observed anti-HCV therapy in a population receiving opioid substitute therapy from community pharmacy. Int J Drug Policy 2017. [Epub ahead of print] doi: 10.1016/j.drugpo.2017.05.042
[41] HCV Action. Diagnosing viral hepatitis in the community: a 3-month pharmacy testing pilot. 2010. Available at: http://www.hcvaction.org.uk/resource/diagnosing-viral-hepatitis-community-3-month-pharmacy-testing-pilot (accessed November 2017)
[42] BMJ Best Practice. Hepatitis C. Br Med J - Best Pract 2017. Available at: http://bestpractice.bmj.com/best-practice/monograph/128/treatment/guidelines.html (accessed November 2017)
[43] Mackridge AJ, Gray NJ & Krska J. A cross-sectional study using freedom of information requests to evaluate variation in local authority commissioning of community pharmacy public health services in England. BMJ Open 2017;7(7):e015511. doi: 10.1136/bmjopen-2016-015511
You might also be interested in…
Genomics surveillance programme for hepatitis C launched by UK Health Security Agency

How we treated more than 100 hepatitis C patients at pharmacist-led clinics in North Wales