This content was published in 2011. We do not recommend that you make any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Key points
- When presented with a patient who has difficulty with swallowing solid dose formulations determine whether he or she is at risk of aspirating or choking and what exactly can be swallowed with respect to texture. Taste may also be a significant issue, particularly in children.
- If possible, observe medicine taking because the technique may be at fault and a simple lesson in how to swallow correctly may be all that is necessary.
- The need for the medicine and whether alternative formulations are available should be considered together with the medical practitioner. Liability should be a consideration and licensed medicines should generally be recommended before unlicensed alternatives.
- Specials are usually expensive so formulation tampering is increasingly being recommended by primary and secondary care trusts. However this should not be recommended if efficacy and safety might be affected.
- Always consider the reasons for the use of special coatings on tablets or in capsules before recommending altering them.
- Always involve the prescriber in any decisions to supply unlicensed medicines or recommend administration outside of its licence.
Tablets and capsules are the most commonly prescribed formulations because they are relatively cheap to mass produce. They do not include water (which can increase the degradation of active ingredients), they can be coated to change absorption characteristics and they are usually convenient for the patient, both to take and transport. However, inability to swallow tablets and capsules is a common problem, and the need to identify alternatives and to consider the effect of tampering with tablets and capsules brings pharmacists’ expertise to the fore.
What is the problem?
Swallowing is a neuromuscular mechanism that occurs in three phases. The oral phase involves voluntary chewing and formation of a bolus and propulsion of that bolus to the pharynx. During this phase, the lips, teeth, tongue, and jaw muscles mix food with saliva to create the proper consistency for ingestion.
Once a bolus is formed it is propelled into the oropharynx by the tongue. At this point (the pharyngeal phase) the bolus is transferred past the larynx and into the oesophagus. In order for this to proceed correctly, several things happen by reflex. Food and liquid do not enter the nose because the soft palate tenses to separate the oral and nasal cavities. The vocal cords and epiglottis close off the trachea (so food does not enter the lungs), and the muscles of the throat contract and the upper oesophageal sphincter relaxes.
The oesophageal phase involves transport of the bolus through the oesophagus, past the lower oesophageal sphincter, and into the stomach.
Swallowing difficulties can arise from either psychological aversion, which can occur at any age, or from dysphagia, which is a physical impairment in the swallowing process. Dysphagia can be acute, for example, in patients with a sore throat or those with an exacerbation of gastro-oesophageal reflux disease, or chronic, for example, in patients who have experienced a stroke, patients with Parkinson’s disease or Huntingdon’s chorea, or those with muscular dystrophy.
Some form of swallowing difficulty is believed to be found in between 70 and 90 per cent of the elderly. This can result from a reduction in the production of saliva and weakening of the muscles involved in swallowing. Patients with oral or oesophageal cancer also, understandably, commonly report difficulties in swallowing.
With the relatively high prevalence of dysphagia in the elderly and its presence in patients with chronic disabling conditions, it is not surprising that the extent of dysphagia has been found to be approximately 15 per cent in general care homes for the older person and up to 30 per cent in hospices1.
According to the Multiple Sclerosis Society, between 30 and 40 per cent of people with multiple sclerosis experience difficulties with swallowing at some time. These difficulties can involve the voluntary or involuntary aspects of swallowing, or both. Early signs of difficulty include:
- Problems chewing;
- Difficulty moving food through the mouth;
- Food sticking in the throat or slow movement down;
- Food or drink coming back up;
- Coughing or spluttering during or after eating;
- Excessive saliva.
The two main concerns with dysphagia are aspiration and choking. In aspiration, the patient has lost the ability to synchronise the closure of the epiglottis and, consequently, foods or liquids pass into the lungs, which have minimal defence systems against boluses containing bacteria. Repeated aspiration of foods and liquids is believed to be associated with pneumonia.
Choking occurs when either the tongue cannot create an appropriate size bolus before swallowing or the throat muscles are too weak to propel boluses into the oesophagus, or both.
As well as having consequences for drug treatment, in the long-term dysphagia can lead to dehydration and malnutrition.
The first stage in identifying solutions is to determine the extent of the dysphagia. Panel 1 describes how swallowing is assessed and how this affects prescribing.
Panel 1: How swallowing should be assessed
Speech and language therapists are usually the most appropriate professionals to assess swallowing. This can be by a brief bedside assessment or can involve more invasive procedures, such as via a nasogastric camera (fibre optic endoscopic examination; FEES) or an X-ray of the swallow of a radio-opaque liquid material (videofluoroscopy).
The assessment should determine whether the oral route is appropriate and, if so, the optimal texture of administered foods and medicines. For example, for patients at risk of aspirating, thicker liquids that hold together are preferred while thin liquids are to be avoided. In comparison, in patients at risk of choking, the texture and size of boluses is important and thinner liquids are more appropriate. In terms of medicines, generally, syrups, emulsions and linctus tend to be thicker than liquids. Determining the extent and reason for the dysphagia is important and should inform pharmacy recommendations.
Psychological aversion to tablet taking can occur at any age so it is important to ask all patients, either during medicines use reviews or on hospital admission if they have any difficulty taking solid dose formulations. Prescribing solid dosage forms for patients who cannot or will not swallow will ultimately affect adherence.
Giving advice on administration
If the oral route is not available it is likely that the patient will be receiving nutrition and liquid either intravenously or via enteral feed tubes (see Panel 2). Intravenous administration of nutrition and medicines is largely limited to acute use because of concerns regarding both localised and centralised infections.
Where patients are able to use the oral route the pharmacist has three main options:
- Review the ongoing need for the medicine (the pharmacist, together with the prescriber, should determine if the medicine is effective and, if so, whether its benefits outweigh any risks);
- Identify another route of administration that does not require the patient to swallow;
- Identify a formulation that can be swallowed.
If the dysphagia is likely to be acute, medicines may be temporarily stopped until the swallow reappears.
Panel 2: Feeding tubes
Enteral feeding tubes, which can be used long-term and are increasingly being used in patients at home, bypass the throat and deliver food, liquids and medicines directly into the stomach or duodenum. Advice for administration of medicines via this route is specialised and two textbooks designed specifically for this purpose are currently available in the UK (see Resources). Pharmacists who are asked to advise on the administration of medicines via such routes and who do not have access to these publications are strongly recommended to contact their regional medicines information centre for advice (details at www.ukmi.nhs.uk).
The administration of medicines via enteral tubes is usually unlicensed because most medicines have not been tested for administration via this route. However, the routine recommendation within both textbooks to crush or disperse tablets or to open capsules before administration should not significantly increase the liability associated with medication in enterally fed patients because it is frequently the only option available. Although the texture and taste of the resultant dispersed or crushed mixtures is theoretically of less concern, patients with enteral tubes do report tasting medicines administered via this route.
Alternative formulations
Formulations that avoid the need to swallow (e.g., patches and buccal formulations, such as fast tabs and melts) are increasingly being made available and these may be both appropriate for and acceptable to some patients.
Traditionally, liquid medicines would have been the first choice alternative in patients who retain some ability to swallow2, but due to recent unprecedented inflation in the cost of liquid specials3 hospitals and primary care trusts are now looking carefully at all such prescriptions. This focus on cost is understandable now and local guidance with respect to liquid medicines and specials in particular should be taken into account when considering potential solutions for a patient.
Due to the complexity of developing and producing a product with a reasonable shelf life, a taste and texture that is acceptable to patients and a sufficient profit margin in a relatively small market, licensed liquid medicines are only available for a limited number of drugs. The unit cost for licensed liquids is, therefore, high compared with that for tablets and capsules and this should be considered when identifying suitable alternatives. The price of licensed liquid medicines is, however, regulated and choosing licensed medicines carries less risk than recommending administration of medicines in an unlicensed form.
Many drugs are available as unlicensed liquids but the quality of the product, patient acceptability, shelf life and cost vary considerably. If specials are to be used then it is always appropriate to confirm the intent with the prescriber (i.e., make them aware of the potential cost and risks). It is also best to source the same product for the patient and, if possible, to obtain batch produced specials, with a certificate of analysis, rather than bespoke specials with a certificate of conformity — the former being exposed to greater levels of quality assurance testing.
When supplying unlicensed medicines pharmacists shoulder increased liability. Where licensed medicines are used within the terms of their licence, liability for any subsequent patient harms falls to the manufacturer. If, however, a patient is harmed by an unlicensed medicine the liability is shared between the prescriber and supplier — the actions of both would be carefully considered in any subsequent legal action.
Manipulating solid dosage forms
If a liquid medicine is unavailable or too expensive then the only remaining option is to consider manipulating tablets and capsules before administration. Although, in many cases, formulation tampering by tablet crushing or dispersing is unlikely to cause any significant harm to the patient, evidence supporting this rational belief (based on a scientific understanding of the relative stability of the active ingredients) is currently limited.
However, there are also some formulations with which it would generally considered to be unwise to tamper.
Modified release preparations
Modified release products generally contain a larger dose of the drug than would be recommended as a single standard release bolus and consequently anything that damages the release mechanism could increase the likelihood of adverse events. In addition, quicker release may result in faster metabolism and a period between doses when levels are subtherapeutic (see Figure).
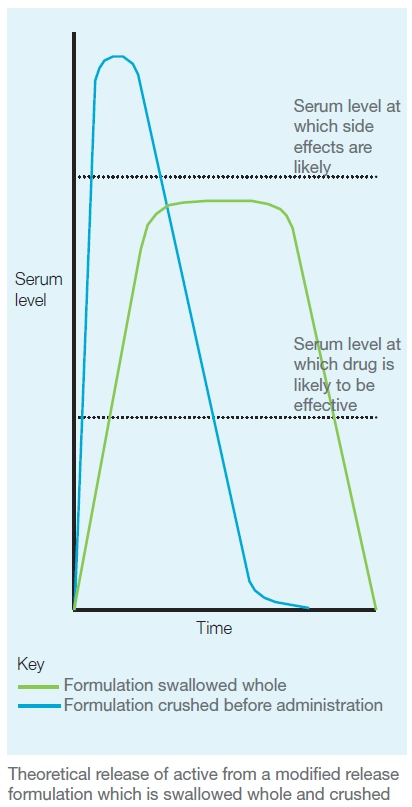
Although, as a general rule, pharmacists should therefore not recommend formulation tampering of modified release products, it is incorrect to state that it is always inappropriate. Some capsules contain modified release pellets that can be safely taken from the outer shell of the capsule and, provided these are not tampered with, release properties will not be affected. For example, modified release morphine capsules, such as MXL, can be opened and their contents sprinkled on food. However, pharmacists must understand the release mechanism before recommending emptying out capsules. For example, for Madopar CR the capsule itself forms the sustained released element so the contents should not be removed.
Enteric coating
Enteric coats are placed on tablets to protect the stomach from the drug or the drug from the stomach, or to release the drug beyond the stomach in the gastrointestinal tract. Although the evidence for the effectiveness of enteric coats for protecting the stomach is limited, coating to protect ingredients such as omeprazole and lansoprazole from stomach acid is known to be effective. It is, therefore, reasonably appropriate never to recommend tampering with enteric coated formulations.
Film coating
Film coats are used for a variety of reasons, which include providing protection against moisture in the environment, making the medicine easier to swallow, masking the taste of the drug within the medicine or preventing contact sensitisation when handling the medicine. The reasons for film coating a tablet should be ascertained before recommending that is it crushed or dispersed. In many cases this practice will not adversely affect patient care although, where the coating is there to mask taste, a crushed or dispersed tablet may be unpalatable.
Other cautions
It is unsafe to recommend that hormonal products, such as finasteride, or medicines where contact is undesirable, such as methotrexate, are manipulated by patients or carers. Aerosolisation of small particles means that these products can be ingested. In addition, direct contact can cause contact sensitisation. Consequently when considering the appropriateness of tablet crushing or dispersing any potential danger to the administrator should be identified.
Medicines with narrow therapeutic windows can switch from efficacious to problematic (creating adverse events) or even dangerous, through minimal changes to bioavailability. For example, crushing or dispersing digoxin tablets, which have a bioavailability of 70 per cent, could in theory increase this to 100 per cent, which would almost increase the dose received by 50 per cent.
It would be inappropriate never to allow formulation tampering in medicines with narrow therapeutic windows but in such instances the patient should be monitored for effects, in terms of drug efficacy and side effects.
A point to remember when asked for advice on how best to administer medicines to a patient who cannot swallow is that only prescribers can authorise unlicensed use of medicines. Pharmacists without prescribing rights considering recommending formulation tampering should ask the prescriber to authorise the activity in writing, preferably as a direction on any prescription4.
Special considerations for children
Many myths surround swallowing in children and it is important that pharmacists understand children’s issues well enough to be able to provide parents and carers with adequate medicines management advice.
The swallow reflex can be viewed antenatally and most full-term babies are able to swallow effectively from day one. The size of the larynx and pharynx within newborns and young children usually predicates a need for liquid medicines. However, there is frequently no physiological reason why older children cannot be given tablets or capsules to take and many may prefer this if given the option.
Some older children might have an aversion to taking tablets and capsules, but it should not be assumed that children must always be prescribed a liquid medicine. There is little definitive information in the literature but it is generally thought that solid dosage forms are acceptable to school age children and certainly by the age of seven or eight years.
Nevertheless, we know from practice that liquids are given to many children and reasons for this include that:
- The child has always been on liquids from a young age and the product remains the same;
- The paediatric dose does not come in a solid dose form;
- The child believes he or she cannot swallow because he or she has had liquid medicines in the past.
There are aspects to pharmaceutical care that should be considered with each of these reasons. The child’s dose should be regularly reassessed, not only to ensure that it is still appropriate for his or her age but also to make sure that it cannot now be rounded to a suitable solid dose form. It should be remembered that many adult doses are acceptable for children greater than 12 years (but always be sure).
Tablets and capsules
Solid dose forms are not only more acceptable in terms of being convenient, they are often more acceptable for taking in front of peers (as the child becomes a teenager). There is also some in-built safety in giving tablets rather than liquids because the dose is controlled — it does not depend on the accuracy of measuring out an amount.
Encouraging children to swallow tablets can be simple or difficult, but most will be able to do so with relative ease over a short time. Teaching children to swallow tablets often takes the form of getting them to swallow something they like (e.g., Smarties) and then switching to small tablets or capsules. Other tips are given in Panel 3.
Even four- and five-year olds often swallow tablets if they are on long-term medication. A classic example is medication in children with attention deficit hyperactivity disorder. Some manufacturers (especially those that do not recommend their capsules are opened) have leaflets to support medication. Various behavioural techniques for children on long-term medication have been successful, including:
- Verbal praise (giving enthusiastic praise if the child swallows successfully);
- Positive reinforcement (rewarding compliance with a sticker);
- Ensuring medicines are given at a consistent time and location and by the same person (so a familiar routine is developed).
If a child fails to swallow, corrective feedback (where efforts are still praised and the child is told what was done well and not so well) can be given.
It should be noted that if only part of a tablet is required and a crushed tablet is to be put into liquid, the contents are unlikely to be evenly spread. Most tablets do not fully dissolve so if a 50mg tablet has been put into 5ml of liquid, there is unlikely to be 10mg in the 1ml.
Panel 3: Tips for giving solid dosage forms to children
- Give the child a spoonful of yoghurt, apple sauce or mousse along with the tablet or capsule so they can swallow it all together.
- Tell the child to put the tablet or capsule on the back of the tongue, take a sip of their favourite drink, then tilt back their head and swallow.
- Get the child to put the tablet or capsule in their mouth and then drink a glass of water through a straw — many children will concentrate on the straw and not think about the tablet or capsule, so it goes down easily.
- Get the child to take a couple of swallows of milk (making the tongue and throat more slippery) before taking the tablet or capsule.
- Put the tablet or capsule onto the child’s tongue then get them to fill their mouth with water (so that his cheeks puff out) and then ask them to swish it all around and swallow.
- Get the child to chew a piece of bread or a biscuit and put the tablet or capsule in their mouth just before they would swallow the bolus.
- Get the child to practise swallowing smaller things first, before switching to a tablet.
* Adapted from Iannelli V. Pill swallowing tips for kids with ADHD. http://pediatrics.about.com
Liquids
Where liquids are required, compliance is greatly affected by palatability (taste and texture). Being able to tell children the flavour of a medicine is always a good start. Methods of masking the taste, if not liked, are always a good start. Strong blackcurrant juice is often a good masking agent and taking it immediately after the medicine or mixed with the medicine (if appropriate) can be helpful.
We have all come across worried parents whose babies have spit out their medicine. How do you ensure the child has had enough? And would it be dangerous to try to give another dose? Oral syringes have definitely made giving medicines easier, resulting in less spillage. Directing the end of the syringe to the back of the cheek and injecting slowly makes it much harder for a baby to spit out than when a spoon is used. However, this does not always work and nothing beats making sure the baby is in a good mood and giving something that he or she likes the taste of.
Pharmacists should note that sugar free liquids are recommended in the National Service Framework for Children and should be sought for all long-term medicines. However, large quantities of sorbitol, which is used as a sweetener in a lot of medicines, can cause diarrhoea.
If taste or volumes are an issue, use of a stronger liquid, if available (e.g., paracetamol suspension 250mg/5ml rather than 120mg/5ml even if the child is younger than six years) can be considered.
The supply of quality liquids is more important for children than for most adults. Not all excipients are appropriate for infants. For example, giving 5ml of Phenobarbital 15mg/5ml Liquid BP to a 3kg baby is about the same as giving an adult a glass of wine with each dose. Alcohol is used in medicines for various reasons — sometimes to improve solubility (the solubility of phenobarbital in water is about 1mg/ml compared with around 100mg/ml in alcohol) and other times as an antimicrobial or for flavouring.
When supplying liquid medicines pharmacists should also ensure that carers know how to use liquid measures appropriately and that those they supply are appropriate for the volumes being measured.
Practice points
1. Look at your top five most used unlicensed oral preparations (specials, imports or crushing tablets) and re-evaluate them (against any alternatives) to see if you are providing the most suitable preparations.
2. Discuss the supply issues of liquid preparations with a local GP, in or der to share learning on best ways of prescribing for those with swallowing difficulties.
3. Now that you are aware of groups of patients who commonly experience dysphagia, make sure these patients are happy taking their tablets and capsules.
Resources
• Swallowing Difficulties (www.swallowing difficulties.com) aims to help people make more informed decisions when taking, administering or prescribing medicines for patients with swallowing difficulties.
• Further information in “Clinical management of swallowing disorders” by Murray T and Carrau R (2006: Plural Publishing; Oxford).
• White R, Bradnam V (editors). Handbook of drug administration via enteral feeding tubes. The Pharmaceutical Press 2010. 2nd Edition.
• Smyth JA. The NEWT guidelines for administration of medication to patients with enteral feeding tubes or swallowing difficulties. North East Wales NHS Trust 2010.
• “Information and guidance on the prescribing and use of unlicensed pharmaceutical specials”, produced by East of England NHS Collaborative Procurement Hub, suggests alternatives to drugs commonly prescribed as specials. It is available at www.eoecph.nhs.uk.
• The Royal Pharmaceutical Society’s best practice guidance for the procurement and supply of pharmaceutical specials is available at www.rpharms.com.
References
1. Wright D. Medication administration in nursing homes. Nurs Stand 2002;16:33–8.
2. Wright D, Chapman N, Foundling-Miah M, Greenwall R, Griffith R, Guyon A, Merriman H. Consensus guideline on the medication management of adults with swallowing difficulties London: Connect Medical 2006.
3. Paduano M. Drugs “specials” cost NHS £160m. Available at www.bbc.co.uk (accessed on 25 September 2010).
4. Griffith R & Davies R. Tablet crushing and the law: the implications for nursing. Prof Nurse 2003;19:41–2.