Life In View / Science Photo Library
Dysphagia is the term used to describe a swallowing disorder usually resulting from a neurological or physical impairment of the oral (mouth), pharyngeal (upper throat) or oesophageal (lower throat) mechanisms[1]
.
Swallowing is a mechanism that allows eating and drinking, which in healthy individuals, is a safe and effective means of maintaining life[2]
. For a normal swallow to occur, food or fluid enters the mouth and is size-reduced through chewing, mixing with saliva to form a bolus that is transferred to the back of the throat with the assistance of the tongue[1]
. The opening of the larynx closes and the ‘bolus’ is propelled into the stomach through the oesophagus[1]
. A normal swallow requires the respiratory, oral, pharyngeal, laryngeal and oesophageal anatomical structures to function in synchrony, which is dependent upon the motor and sensory nervous system being intact[1]
.
Which patients are affected?
Dysphagia is a common complication of stroke. Around 152,000 people have strokes each year in the UK, and up to two-thirds of these patients will experience dysphagia[3]
. It is frequently noted as a symptom in frail older people or in individuals with any impairment of oral structure, or respiratory or neurological systems[1]
, and is observed in a large proportion of people with dementia: of the 815,827 people with dementia in the UK[4]
, dysphagia affects two-thirds of them[5]
. In addition, dysphagia occurs in up to a quarter of 900,000 patients with chronic obstructive pulmonary disease (COPD)[6],[7]
, and is often observed in patients with progressive neurological diseases[8]
(e.g. Parkinson’s disease), and head and neck cancers[9]
.
What are the risks of dysphagia?
Dysphagia is associated with aspiration pneumonia, an inflammation of the lungs and bronchial tubes, usually resulting from an infection, that occurs after inhalation of foreign matter. This can lead to poor functional outcomes, such as dehydration, malnutrition, increased length of hospital stay, an increased need for residential care and increased healthcare costs[10]
. Over 50% of patients with advanced dementia[11]
and up to a third of patients with stroke will develop pneumonia[12]
. Therefore, dysphagia significantly affects quality of life and places patients at risk of pneumonia, malnutrition and death[13]
. While stroke is the third most common cause of death and the most important cause of long-term disability, most stroke-related deaths result from medical complications of the stroke (e.g. 30% of post-stroke deaths are caused by pneumonia) rather than directly because of neurological damage[14]
.
Guidance from the National Institute for Health and Care Excellence (NICE), England’s health technology assessment body, recommends the avoidance of aspiration pneumonia as part of initial stroke patient management[15]
. For patients with dysphagia, NICE recommends that fluids and food should be administered in a form that can be swallowed without aspiration, following specialist assessment of swallowing by a speech and language therapist (SLT)[15]
.
How is dysphagia identified?
Dysphagia is commonly identified when patients cough on intake of food or fluids (audible aspiration). However, some patients, especially post-stroke, aspirate (where food, fluid or saliva enters the airway) without coughing (silent aspiration). Dysphagia in these patients is more difficult to identify and these patients are more likely to develop aspiration pneumonia. In hospital, tools such as video fluoroscopy (VFS), fibre-optic endoscopic evaluation of swallowing (FEES) and cough reflex testing (CRT) are used to identify silent aspiration[16]
. Where dysphagia is not identified and managed, it can lead to significant negative consequences, which range from unintended non-adherence, to choking, aspiration pneumonia and death[1]
.
Dysphagia and medication
Patients with dysphagia are unable to take some oral formulations of medication. In hospital, the formulation of medication for patients with dysphagia is often based on the recommendation of the SLT who reviews the patient ability to swallow fluid and food. Pharmacists need to understand appropriate fluid consistency and food texture in order to advise patients on the safest and most efficacious formulation, minimising the risk of aspiration pneumonia and choking.
A patient may also present to their local community pharmacy and, therefore, the pharmacist may be required to first identify whether the patient is having difficulties swallowing medication. This is an opportunity to establish whether the patient has difficulty swallowing food or liquids, and whether they have experienced any associated weight loss. A patient with these symptoms can then be referred via GP services to community speech and language services.
Health professionals are often unaware of patients with dysphagia, as the patient, their carers or family may alter formulations of medication to facilitate swallowing. Medication administration errors have been identified more than three times as frequently in patients with dysphagia than in those without[17]
. The administration of crushed tablets and the contents of opened capsules exposes the patient to the unpleasant taste and potentially reduced efficacy of the medication[18]
, as well as having legal implications for professionals who administer medicines off-label[19]
.
What can a patient with dysphagia swallow?
Many healthcare professionals assume that patients with dysphagia cannot swallow solid oral formulations (whole tablets and capsules) and need to be prescribed liquids. This is a misconception; patients must be assessed according to the degree of dysphagia. For example, some thinner liquid medicines can increase the risk of coughing and aspiration in a patient with dysphagia[20]
. To facilitate a safer swallow and slow oesophageal transit, medication may need to be formulated as thicker fluids or mixed with more textured food.
The National Patient Safety Agency (NPSA), which leads and contributes to improved, safe patient care in the UK and now undertakes similar functions under NHS England, has developed the dysphagia diet food texture descriptors so that healthcare professionals and food providers can use common language and standardised terminology to describe dysphagic diets[21]
.
The food textures are:
- B = thin purée dysphagia diet;
- C = thick purée dysphagia diet;
- D = pre-mashed dysphagia diet;
- E = fork-mashable dysphagia diet.
Thickened fluids are described as:
- Stage 1: syrup consistency, should pour like single pouring cream;
- Stage 2: custard consistency, should drop easily off a teaspoon rather than pour;
- Stage 3: pudding consistency, should stay on a spoon like well-whipped double cream.
Guidance is also available on the thickening of fluids[22]
,[23]
. Various commercial brands of thickeners provide instructions on how to amend fluids to provide the desired stage. There is less likely a drug-thickener interaction if a starch-based thickener (e.g. Hormel’s ‘Thick and Easy Powder’) is used, as starch is neutral charged compared with negative charged xanthan gum (Resource), which theoretically can impede the drug dissolution process, reducing the release of the drug[24]
.
Figure 1 provides a flowchart that may be useful in determining the type of formulation suitable for a patient with dysphagia. Boxes 1–3 provide both general and specific guidance for managing different degrees of dysphagia linked to the flow chart in figure 1.
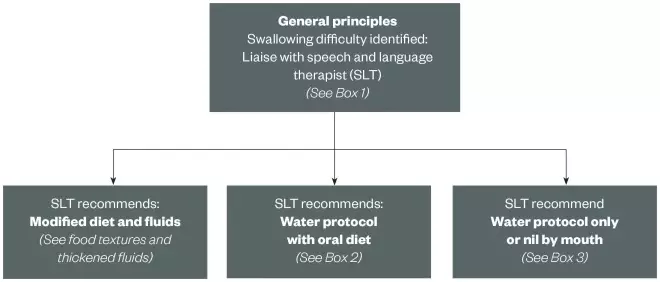
Figure 1: Flowchart to support pharmacists working with patients with dysphagia
Support for optimising formulations
Where possible, tablets and capsules should be administered whole and intact with the appropriate food texture and fluid consistency. Some smaller tablets and capsules can be safely swallowed whole when mixed with a food of a suitable consistency (e.g. within a purée). If the patient has difficulty swallowing a medication whole, liquids can be used but only if thickened to the appropriate consistency. In the absence of a licensed liquid formulation of the appropriate fluid consistency, pharmacists will need to investigate the safety of crushing tablets/opening capsules to be administered with modified food or fluid, for example from the UK Medicines Information (UKMi) resource or the manufacturer. It is important to note that this formulation will be unlicensed. Special order liquid medications (‘specials’) are expensive, unlicensed formulations that have short expiry dates once opened. It should be noted that the fluid consistency of the ‘special’ may be inappropriate for the dysphagic patient and must be assessed prior to administration[19]
.
General principles for medication prescription and review are included in Box 1.
Box 1: Six general principles to consider when prescribing or reviewing medication for a patient with dysphagia
1. Perform a medication review to see if the medication is still indicated and required.
2. Can the tablet or capsule be swallowed whole with yogurt? Compatibility with yogurt or puréed food must be checked for drug–food interaction (e.g. levothyroxine, bisoprolol, ramipril). Remember to check the speech and language therapist’s recommendations regarding diet stages and fluid consistency.
3. Can the tablet be crushed or the capsule opened, and administered with thickened fluid or yoghurt?
4. Can the tablet be swallowed whole when mixed with the appropriate consistency of food or fluid? Check what size of tablet the patient can comfortably swallow. Small tablets, such as those measuring less than 4mm (including bisoprolol and levothyroxine), may be suitable.
5. Does the person administering or the nurse know how to prepare and administer the medication?
6. Communicate with the GP or community pharmacist on the patient’s medication administration and swallow.
It should be noted that if administered with food, medication should always be at room temperature and one tablet or capsule (whole or crushed or contents of capsule) should be administered per spoonful of food. Crushed tablets and the contents of open capsules will alter the taste of food, because solid oral medications are bitter tasting. This can also affect adherence – medication may be refused if it tastes unpleasant.
Legal implications
Under the Medicines Act 1968, which governs all medicine usage in the UK, all prescribers are allowed to prescribe medicines outside of their licence either for unlicensed patient groups (off-label) or medicines with no licence (e.g. medicines with a non-EU licence, [unlicensed]). By prescribing an off-label or unlicensed medicine, the liability rests with the prescriber[25]
. It may also rest with the administrator (nurse or carer) and supplier if they are aware of the unlicensed or off-label use and the person was in a position to intervene[26]
.
Prescribing decisions that fall below the accepted standard can lead to civil, criminal and professional liability, as well as breach of an employment contract. The modification of oral medications should be discussed with the patient and documented in their care plan. The patient’s medication should be clearly labelled and the instructions should clearly state how the medicine is to be administered. If the oral dosage form is altered, it must be done immediately before administration.
Advice for patients and carers
Patients and carers should be aware that the medication being administered (e.g. a crushed tablet in food) is not designed to disguise the medication but to aid the patient swallowing it. Figure 2 provides guidance on crushing tablets for community pharmacists.
Further information to support safe administration of medicines for patients with dysphagia can be found under ‘Useful resources’.

Figure 2: Example of directions of how to crush tablets for community care
Source: With permission from Lelly Oboh, Consultant Pharmacist on behalf of Lambeth Community Health
Box 2. Water protocol with oral diet
- Medications not to be taken with water. Pharmacy to advise on medication administration.
- Check food texture being given to the patient with a speech and language therapist as medication may be given with food.
- No water to be given at meal times or for 30 minutes after eating.
- No other fluids to be given (i.e. tea, orange juice); water only.
- No thin food textures (i.e. soup, ice cream, cereal and milk).
- Do not give thickend fluids, even if the patient is coughing.
- Regular mouth care to keep the mouth clean, especially after meal times.
Remember that the general principles (Box 1) still apply.
Box 3. Nil-by-mouth and water protocol with no oral diet
Managing patients on water protocol with no oral diet:
- Medications must not be taken with water or any oral food.
- Is an enteral feeding tube to be inserted? If yes, discuss with pharmacy to amend and advise on the most appropriate formulations (see ‘Handbook of drug administration via enteral feeding tubes’[26]
and ‘NEWT guidelines’[27]
). - Only water to be sipped when required.
- No other fluids to be given (i.e. tea, orange juice); water only.
- Regular mouth care to keep the mouth clean, especially after meal times.
Managing patients with enteral feeding tubes:
- If the patient has an enteral feeding tube, refer to ‘Handbook of drug administration via enteral feeding tubes’[26]
and ‘NEWT guidelines’[27]. - Review cost and availability of medication of licensed liquid/dispersable tablet. Try to avoid unlicensed crushing of tablets, opening of capsules or unlicensed special liquid formulation. Full guidance is available here[25]
. - Regular mouth care to keep the mouth clean.
- Annotate a discharge letter to the patient’s GP and communicate with a community pharmacist on the patient’s medication administration, rationale for choice and length of therapy.
Useful resources
- NHS East & South East England Specialist Pharmacy Services. Supporting patients with swallowing difficulties: medicines and dysphagia.
- Guidelines in practice. Consensus guideline on the medication management of adults with swallowing difficulties.
- Resource clinical. Crushing tablets and drug administration via enteral feeding tubes.
- Medicines complete. Handbook of drug administration via enteral feeding tubes.
- Blackpool teaching hospitals NHS trust. Thickened fluids and modified diet – speech and language therapy.
- Medicines optimisation in patients with dysphagia.
- NEWT guidelines.
- Royal Pharmaceutical Society. Guidance on pharmaceutical issues when crushing, opening or splitting oral dosage forms. June 2011.
- Scottish Intercollegiate Guideline Network (SIGN). Management of patients with stroke: identification and management of dysphagia.
- UKMi. What are the therapeutic options for patients unable to take solid oral dosage forms? UKMI Q&A 294.3
‘How we improved dysphagia care and saved more than £40,000 per year‘, The Pharmaceutical Journal
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Royal College of Speech and Language Therapists (RCSLT) resource manual for commissioning and planning services for SLCN: Dysphagia 2009 (updated 2014). Available at: http://www.rcslt.org/speech_and_language_therapy/commissioning/dysphagia_manual_072014 (accessed July 2016)
[2] Wilkinson S, Rowe G & Lambert N. The risks of eating and drinking. EMBO Rep 2004;5(Suppl 1):S27–S31. doi: 10.1038/sj.embor.7400225
[3] Townsend N, Wickramasinghe K, Bhatnagar P et al. Coronary heart disease statistics 2012 edition. British Heart Foundation: London.
[4] Dementia UK: Update 2014. Available at: https://www.alzheimers.org.uk/site/scripts/download_info.php?fileID=2323 (accessed June 2016)
[5] Steele C, Greenwood C, Ens I et al. Meal time difficulties in a home for the aged: not just dysphagia. Dysphagia 1997;12:43–50. doi: 10.1007/PL00009517
[6] Healthcare Commission. Clearing the air: a national study of chronic obstructive pulmonary disease. 2006. London: Healthcare Commission.
[7] McKinstry M, Tranter M & Sweeney J. Outcomes of dysphagia intervention in a pulmonary rehabilitation program. Dysphagia 2010;25:104. doi: 10.1007/s00455-009-9230-3
[8] Hartelius L & Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatrica 1994;46(1):9–17. doi: 10.1159/000266286
[9] McCabe D, Ashford J, Wheeler-Hegland K et al. Evidence-based systematic review: oropharyngeal dysphagia behavioral treatments. Part IV—Impact of dysphagia treatment on individuals’ postcancer treatments. J. Rehabili Res Devel 2009;46(2):205–214. doi: 10.1682/JRRD.2008.08.0092
[10] Smithard D, Parks C, Morris J et al. Complications and outcome after acute stroke: does dysphagia matter? Stroke 1996;27:1200–1204. doi: 10.1161/01.STR.27.7.1200
[11] Mitchell SL, Teno JM, Kiely DK et al. The clinical course of advanced dementia. N Engl J Med 2009;361(16):1529–1538. doi: 10.1056/NEJMoa0902234
[12] Sellars C, Bowie L, Bagg Jet al. Risk factors chest infection in acute stroke. Stroke 2007;38(8):2284–2291. doi: 10.1161/STROKEAHA.106.478156
[13] National Patient Safety Agency. Problems swallowing, resources for healthcare staff. July 2007. Available at: http://www.nrls.npsa.nhs.uk/resources/?entryid45=59823 (accessed May 2016)
[14] Heuschmann PU, Kolomonsky-Rabas PL, Misselwitz B et al. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med 2004;13(16):1761–1768. doi: 10.1001/archinte.164.16.176 1
[15] National Institute for Health and Care Excellence (NICE) Clinical Guideline 68. Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). July 2008. Available at: https://www.nice.org.uk/guidance/cg68 (accessed May 2016)
[16] McFarlane M, Miles A, Atwal P et al. Interdisciplinary management of dysphagia following stroke. British Journal of Neuroscience Nursing 2014;10(1):267–272.
[17] Kelly J, Wright D & Wood J. Medication errors in patients with dysphagia. Nursing Times 2012;108(21):12–14. Available at: http://www.nursingtimes.net/Journals/2012/05/18/i/j/b/120522-med-errors.pdf (accessed June 2016)
[18] Royal Pharmaceutical Society. Pharmaceutical issues when crushing, opening or splitting oral dosage forms. June 2011. Available at: http://www.rpharms.com/support-pdfs/pharmaceuticalissuesdosageformsjune-2011.pdf (accessed June 2016)
[19] UKMi. Medicines Q&A 294.3. What are the therapeutic options for patients unable to take solid oral dosage forms? July 2013. Available at: https://www.evidence.nhs.uk/search?q=therapeutic+options+for+patients+unable+to+take+solid+oral+dosage+forms (accessed July 2016)
[20] Horner J, Massey EW, Riski JE et al. Aspiration following stroke: clinical correlates and outcome. Neurology 1988;38(9):1359–1362. doi: 10.1212/WNL.38.9.1359
[21] National Patient Safety Agency (NPSA). Dysphagia diet food texture descriptors. April 2011. Available at: http://www.thenacc.co.uk/assets/downloads/170/Food_Descriptors_for_Industry_April_2011%5B1%5D.pdf (accessed May 2016)
[22] Scottish Intercollegiate Guidelines Network (SIGN). Clinical Guideline 119. Management of patients with stroke: identification and management of dysphagia. June 2010. Available at: http://www.sign.ac.uk/guidelines/fulltext/119/ (accessed May 2016)
[23] Blackpool teaching hospitals NHS trust. Thickened fluids and modified diet – speech and language therapy. Available at: http://www.bfwh.nhs.uk/wp-content/uploads/2015/08/CPL089.pdf (accessed May 2016)
[24] Cichero J. Thickening agents used for dysphagia management: effect on bioavailability of water, medication and feelings of satiety. Nutrition Journal 2013;12:54. doi: 10.1186/1475-2891-12-54
[25] UK Government. Medicines Act (1968). Available at: http://www.legislation.gov.uk/ukpga/1968/67 (accessed June 2016)
[26] White R & Bradnam V. Handbook of drug administration via enteral feeding tubes. Print version, 3rd edition 2015. Online version available at: http://www.medicinescomplete.com (accessed June 2016)
[27] Smyth J. The NEWT Guidelines for administration of medication to patients with enteral feeding tubes or swallowing difficulties. Print version, 2nd edition published 2012. Online version available at: http://www.newtguidelines.com (accessed June 2016)


