Shutterstock
After reading this article, you should be able to:
- Understand the cycle of hair growth;
- Identify symptoms associated with alopecia areata;
- Understand the causes of alopecia areata and how it may be diagnosed;
- Know the pharmacological and non-pharmacological treatment measures used to manage alopecia areata.
Alopecia areata is an autoimmune condition that affects hair follicles, resulting in hair loss1–5. Alopecia is the Latin term for hair loss, while areata refers to the patchy nature of the hair loss1–5.
Alopecia areata encompasses the common conditions of non-scarring hair loss, including alopecia totalis (i.e. hair loss of the whole scalp), alopecia universalis (i.e. hair loss affecting the whole body, face and scalp), alopecia ophiasis (i.e. hair loss from the sides and back of the scalp), as well as diffuse alopecia areata2,4,5.
Alopecia is non-specific for gender, ethnicity and may occur at any age. Data show that 50% of patients may be diagnosed with alopecia in infancy3–5, while alopecia areata may be inherited in 20% of cases6.
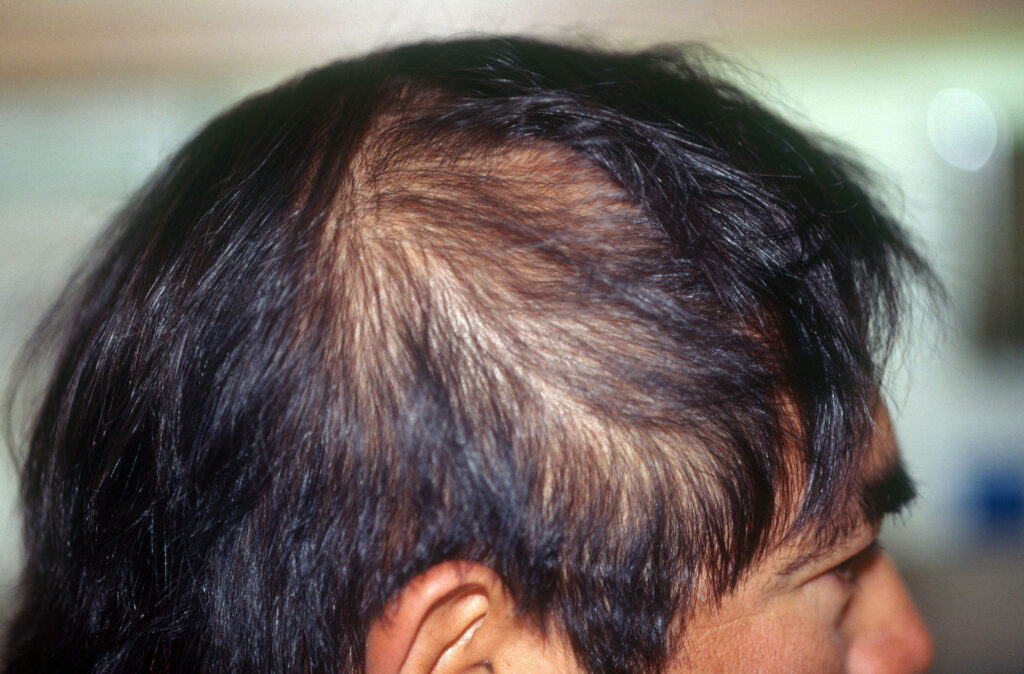
Alopecia areata may vary in severity of hair loss, from small coin-sized bald patches on the scalp to loss of eyebrows, eyelashes, beard, face, scalp and, subsequently, hair loss on the entire body1–5.
As the condition is non-scarring, it means that the roots have not been lost and have the potential to grow back, which varies between patients and with the extent of hair loss. Patients may also still lose their hair once it has grown back1-5.
Androgenetic alopecia, also known as female and male pattern hair loss, differs from alopecia areata, where the hair tufts gradually shrink, resulting in classic ‘M shape’ receding hair line in males and thinning hair at the crown of the head in women. Androgenetic alopecia is a hereditary and hormone-driven condition, namely the androgen dihydrotestosterone (DHT). Increased levels of DHT in hair follicles may lead to shorter cycle of hair growth. Unlike alopecia areata, androgenetic alopecia occurs in 50% of men aged over 50 years and 50% of women aged over 65 years7. Androgenetic alopecia is beyond the scope of this article, which will cover alopecia areata only.
It is important for pharmacists to recognise signs and symptoms of both alopecia areata and androgenetic alopecia, as patients may present to local pharmacies and GP surgeries for advice, and ways to mitigate further hair loss. Products such as minoxidil are available over the counter to manage symptoms of hair loss for both indications, while more novel medications, such as Janus kinase inhibitors, are reserved for alopecia areata alone.
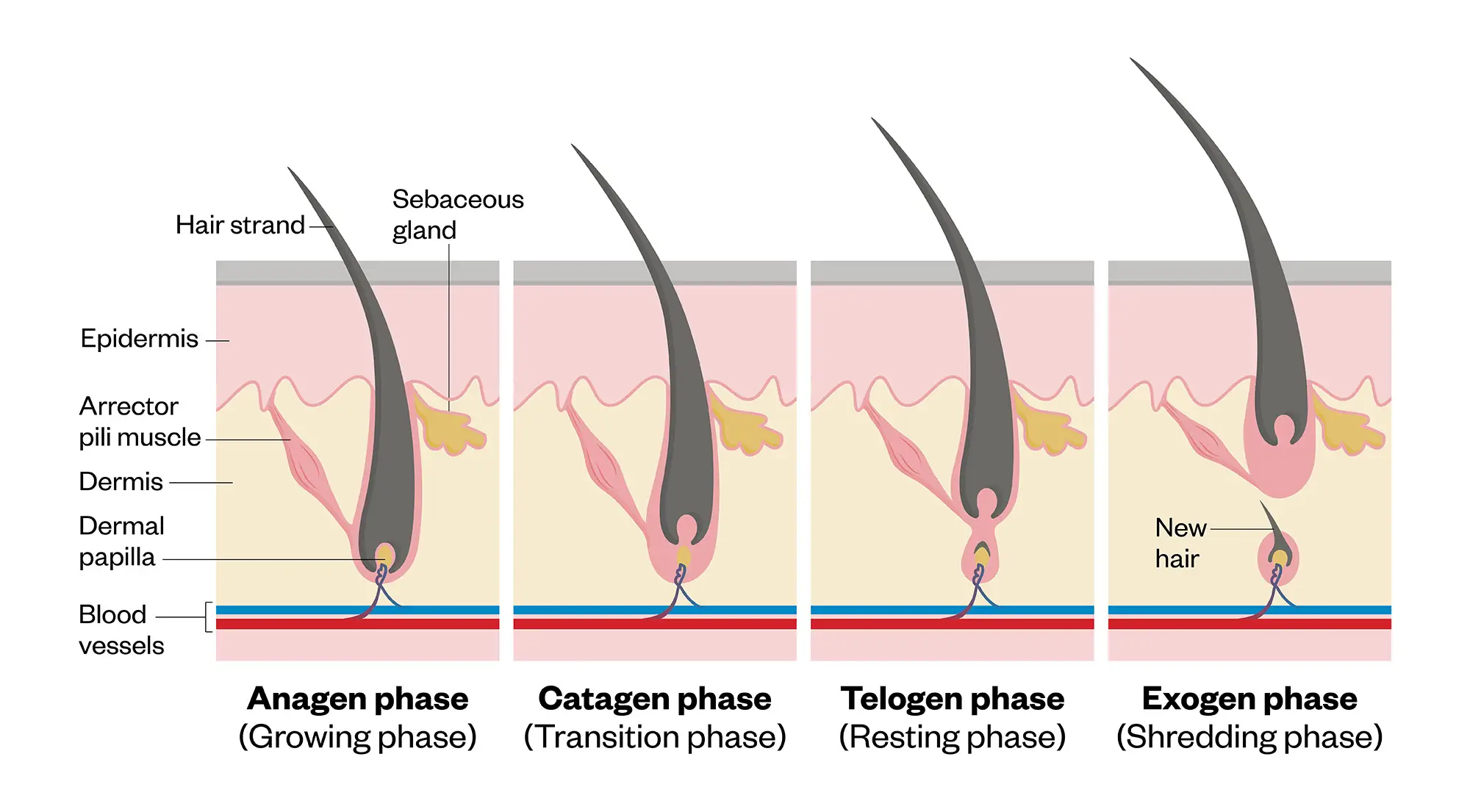
Cycle of hair growth
There are four phases in the hair cycle (see Figure 1). All hair follicles may reside in different stages in the hair cycle and can undergo 10–30 cycles in a lifetime3,5.
Most individuals, on average, will have 100,000 scalp hairs at any time. Normal hair shedding may occur at a rate of 100–150 telogen hairs per day. As some hairs reside in the anagen phase, the total amount of hair should remain stable in healthy circumstances5.
Scalp hair may reside in the anagen phase between two to eight years, and phase length may decline with increasing patient age. Some factors, including hormones (thyroid, DHT), inflammation, stress, nutritional deficiency and poor sleep quality may promote hair to the telogen phase of the hair cycle.
This may be reversed by promoting blood flow to the scalp via scalp massages and medications, such as finasteride and minoxidi, as well as stimulate follicle growth with herbs and supplements, including pumpkin seed oil and latanoprost, as well as infrared light. Growth factors and platelet-rich plasma may also stimulate new follicle development. Apart from topical minoxidil, these therapies are not available in a community pharmacy setting and patients should be referred to their GP to discuss treatment options. It is also important to note that the shedding of anagen hair is uncommon, and patients should be referred to their GP for further investigation3–5,8.
Figure 1: Representation of hair growth cycle
The Pharmaceutical Journal / Shutterstock.com
Causes
There is no known exact cause of alopecia areata; however, several studies have demonstrated that the body’s natural defences mistakenly attack hair follicles, ultimately stopping hair growth. Alopecia areata is predominantly thought to be an autoimmune condition, resulting from T cell activation — specifically, NKGD2+, which prevents the hair follicle from producing more hair3–5.
Individuals with alopecia areata are more likely to experience autoimmune conditions, such as thyroid disease, vitiligo and type 1 diabetes, as well as atopic conditions, such as eczema, asthma and hay fever1–5.
While there are several theories regarding environmental triggers for alopecia areata, such as stress and infections, this has not been proven. Alopecia areata can also be common in patients with chromosomal disorders, such as Down’s syndrome2–5.
It is important to note that alopecia areata cannot spread to other people, with no connection to food or diet1,2.
Symptoms
Alopecia areata is often noticed as sudden patchy hair loss. Some of these patches may grow and eventually join1–5. In some patients, the loss of hair is rapid, and hair may fall without any patches occurring2.
Most patients do not experience any pain associated with hair loss; however, some may notice tingling, itching and burning sensations, as well as headaches. In some cases, pain in the skin may also occur before the hair falls out1–5.
Nail abnormalities are often associated with more severe forms of the disease and may include nail pitting and ridging. Other features may include:
- Koilonychia (i.e. a concave outer surface of the nail);
- Trachyonychia (i.e. brittle nails with longitudinal ridging);
- Beau’s lines (i.e. horizontal ridges);
- Onychorrhexis (i.e. brittleness with breakage);
- Onychomadesis (i.e. the shedding of the nail);
- Onycholysis (i.e. the loosening of the nail), leukonychia (i.e. white patches under the nails); and
- Red spots in the lunula1–4.
Diagnosis
Patients may initially present to local pharmacies or to their GP surgery seeking advice and management of hair loss before being referred to a specialist in dermatology, if appropriate1,2,5.
Patients should be referred to a dermatologist if:
- The diagnosis is uncertain;
- The person affected is a child;
- The affected person is pregnant or breastfeeding;
- The affected person wishes for specialist input or prefers to have their treatment under specialist advice;
- Hair loss does not respond to treatment in primary care1.
Patients may be diagnosed with alopecia areata if there are typical clinical features, as well as if other causes of hair loss — such as fungal infections, trichotillomania (i.e. hair pulling), traction alopecia and scarring alopecia — have been excluded. It is also important to undertake a full medical history and examine patients before a diagnosis of alopecia areata can be confirmed1,2,4.
Figure 2 shows various clinical presentations of alopecia areata9.
Figure 2: Clinical presentations of alopecia areata
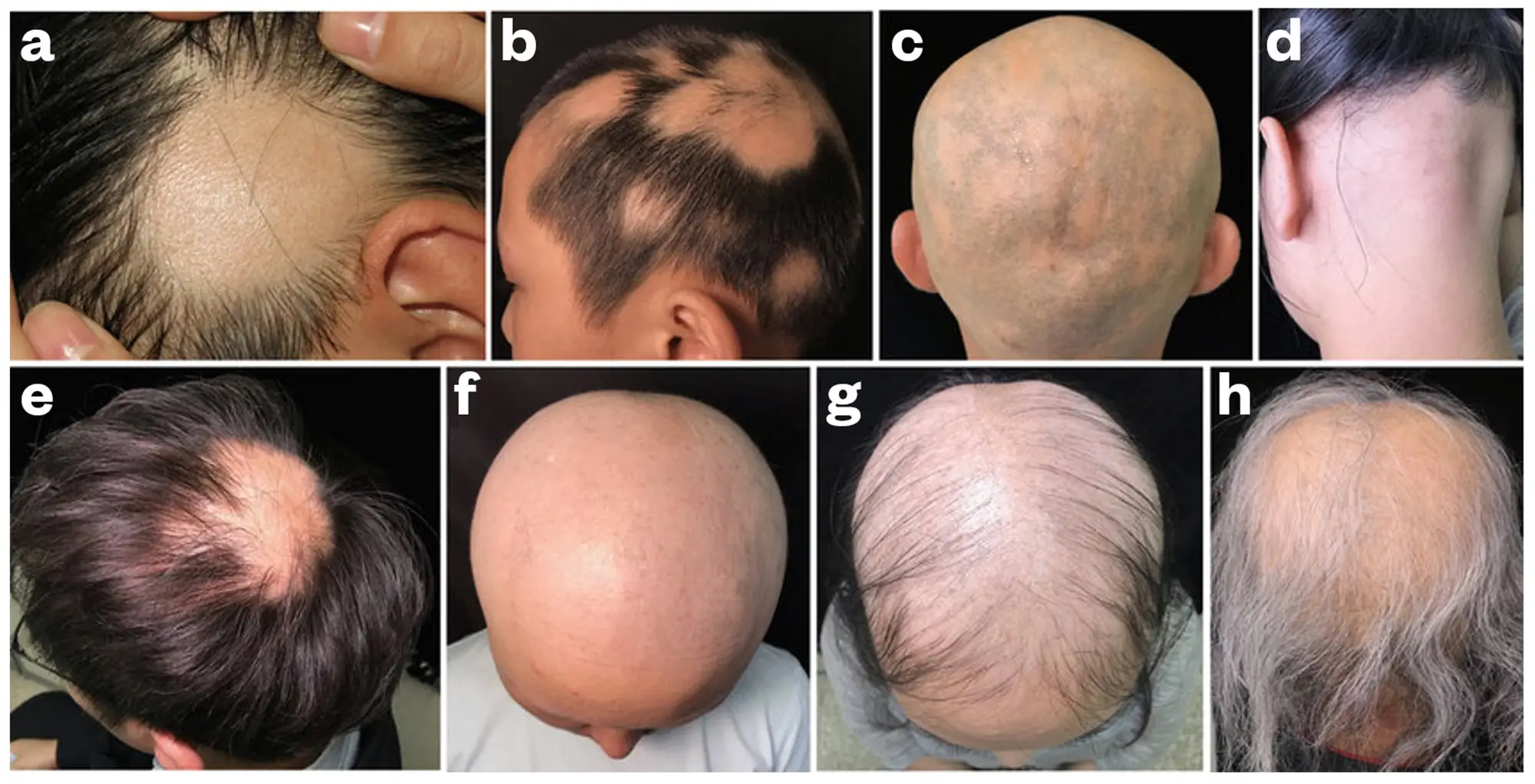
B: Multiple hair loss patches;
C: Reticulate pattern of AA;
D: Ophiasis;
E: Sisaipho;
F: Alopecia universalis;
G: Acute diffuse and total alopecia;
H: Marie Antoinette syndrome (‘overnight graying’)
(Reused with permission)9
Differential diagnosis
Differential diagnosis of disorders that cause patchy hair loss can be seen in Figure 3. In addition, disorders associated with diffuse hair loss can be seen in Figure 410.
Diffuse hair loss
Figure 4: Telogen effluvium
Anagen effluvium is hair shedding owing to the interruption of active or anagen hair growth11. It is non-scarring, diffuse alopecia, which is often an acute result of exogenous or endogenous causes via drugs, including chemotherapy, radiation, toxins or inflammation (e.g. alopecia areata)11.
Management
There are many treatment options available — each with varying levels of effectiveness for different patients, with milder cases often having better treatment outcomes5. Options will be based on the type, severity and duration of hair loss, age of patient, treatments tried and whether atopic conditions co-exist — with the aim of reducing the immune response or stimulating hair regrowth5,12,13. Often treatments are used in combination depending on the severity.
Depending on the patient’s perspective of body image and their coping strategies, the decision not to treat can be made13. For the pharmacological measures available to help manage alopecia aerate, see Table 11–3,12–32.
Non-pharmacological measures
The main aim of non-pharmacological options in alopecia areata is to hide hair loss and improve patient wellbeing. The non-pharmacological treatment options for alopecia areata can be seen in Table 21,2,13,32.
Other options include shaving, microblading, micro-pigmentation and eyebrow stickers — some of which are temporary and non-invasive.
Case study
Figure 5 shows a patient case study, highlighting the patient journey from diagnosis to present time. The case study demonstrates the complexity of management strategies for alopecia and that progress is not linear.
Patient counselling and support
- The psychological impact of hair loss should not be underestimated in patients experiencing hair loss1,2;
- Patients should be advised to protect bald patches from sunlight to prevent sun damage;
- It is important to work with the patient’s expectations, as treatment takes time and hair regrowth may occur without treatment, while hair loss can reoccur despite treatment;
- Promote patient self-care as psychological effect of hair loss has a vital impact on patients’ general health — this includes developing daily habits for emotional wellbeing, building emotional resilience, coping strategies for stress and anxiety, self-compassion and self-understanding5;
- Counselling may be available via the GP to support patient’s wellbeing, as well as local support groups and online forums5.
- 1.Alopecia areata. National Institute for Health and Care Excellence. 2024. https://cks.nice.org.uk/topics/alopecia-areata/
- 2.Alopecia areata. British Association of Dermatologists. 2024. https://www.bad.org.uk/pils/alopecia-areata/
- 3.Alopecia – an overview. Primary Care Dermatology Society. 2024. https://www.pcds.org.uk/clinical-guidance/alopecia-an-overview
- 4.Alopecia Areata. BMJ Best Practice. 2022. https://bestpractice.bmj.com/topics/en-gb/222
- 5.Alopecia Areata. Alopecia UK. https://www.alopecia.org.uk/alopecia-areata
- 6.Types of alopecia. Alopecia UK. https://www.alopecia.org.uk/Pages/Category/types-of-alopecia
- 7.Androgenetic alopecia. Alopecia UK. https://www.alopecia.org.uk/androgenetic-alopecia-pattern-hair-loss
- 8.Natarelli N, Gahoonia N, Sivamani RK. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. JCM. 2023;12(3):893. doi:10.3390/jcm12030893
- 9.Alopecia areata. National Alopecia Areata Foundation. https://www.naaf.org/alopecia-areata/
- 10.Telogen effluvium. DermNet. https://dermnetnz.org/topics/telogen-effluvium
- 11.Anagen effluvium. DermNet. https://dermnetnz.org/topics/anagen-effluvium
- 12.National Alopecia Areata Foundation. National Alopecia Areata Foundation. https://www.naaf.org/
- 13.Harries MJ, Ascott A, Asfour L, et al. British Association of Dermatologists living guideline for managing people with alopecia areata 2024. British Journal of Dermatology. 2024;192(2):190-205. doi:10.1093/bjd/ljae385
- 14.British National Formulary: Triamcinolone acetonide. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/triamcinolone-acetonide/
- 15.British National Formulary: Hydrocortisone butyrate. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/hydrocortisone-butyrate/
- 16.British National Formulary: Mometasone furoate. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/mometasone-furoate/
- 17.British National Formulary: Clobetasol propionate. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/clobetasol-propionate/
- 18.British National Formulary: Prednisolone. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/prednisolone/
- 19.British National Formulary: Methylprednisolone. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/methylprednisolone/
- 20.British National Formulary: Minoxidil. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/minoxidil/
- 21.British National Formulary: Latanoprost. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/latanoprost/
- 22.British National Formulary: Bimatoprost. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/bimatoprost/
- 23.British National Formulary: Dithranol. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/dithranol/
- 24.American Academy of Dermatology Association . American Academy of Dermatology Association . https://www.aad.org/
- 25.Diphencyprone (DCP) Treatment. Royal Devon NHS. 2023. https://www.royaldevon.nhs.uk/media/dcvldmhp/patient-information-leaflet-diphencyprone-dcp-treatment-rde-23-020-001.pdf
- 26.British National Formulary: Tofacitinib. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/tofacitinib/
- 27.British National Formulary: Ritlecitinib. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/ritlecitinib/
- 28.British National Formulary: Baricitinib. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/baricitinib/
- 29.British National Formulary: Dupilumab. National Institute for Health & Care Excellence. https://bnf.nice.org.uk/drugs/dupilumab/
- 30.Ritlecitinib for treating severe alopecia areata in people 12 years and over. National Institute for Health and Care Excellence. 2024. https://www.nice.org.uk/guidance/ta958
- 31.MHRA authorises Litfulo as a treatment for severe alopecia areata in adults and adolescents 12 years and older. Medical and Healthcare Products Regulatory Agency. 2023. https://www.gov.uk/government/news/mhra-authorises-litfulo-as-a-treatment-for-severe-alopecia-areata-in-adults-and-adolescents-12-years-and-older
- 32.Hair loss. NHS. https://www.nhs.uk/conditions/hair-loss/