Viennaslide / Alamy Stock Photo
Undertaking and performing scientific, clinical and practice-based research is only the beginning of the scholarship of discovery[1]
. For the full impact of any research to be achieved and to have an effect on the wider research and scientific community, it must be published in an outlet accessible to relevant professionals[2]
.
Scientific research is often published in peer-reviewed journals. Peer review is defined as the unbiased, independent, critical assessment of scholarly or research manuscripts submitted to journals by experts or opinion leaders[3]
. The process and requirements of reviewers has been covered recently[4]
. On account of this rigorous process, peer-reviewed scientific journals are considered the primary source of new information that impacts and advances clinical decision-making and practice[5]
,[6]
.
The development of a research article can be helpful for the promotion of scientific thinking[7]
,[8]
and the advancement of effective writing skills, allowing the authors to participate in broader scientific discussions that lie beyond their scope of practice or discipline[2]
.
This article aims to provide pharmacists and healthcare professionals who are undertaking research with an understanding of how to produce a research article for publication, as well as points to consider before submission to a peer-reviewed journal.
Importance of the research question
This article will not go into detail about forming suitable research questions, however, in principle, a good research question will be specific, novel and of relevance to the scientific community (e.g. pharmacy – pharmacists, pharmaceutical scientists, pharmacy technicians and related healthcare professionals). Hulley et al. suggest using the FINER criteria (see ‘Figure 1: FINER criteria for a good research question’) to aid the development of a good research question[9]
.
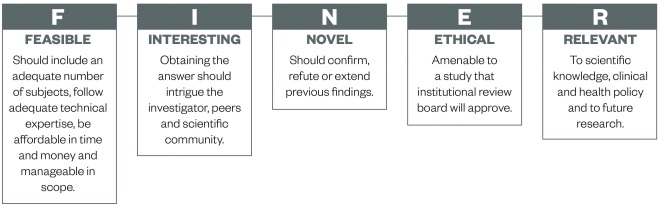
Figure 1: FINER criteria for a good research question
Source: Hulley S, Cummings S, Browner W et al.[9]
The FINER criteria highlight useful points that may generally increase the chances of developing a successful research project. A good research question should specify the population of interest, be of interest to the scientific community and potentially to the public, have clinical relevance and further current knowledge in the field.
Having a clear research question that is of interest to those working in the same field will help in the preparation of an article because it can be used as the central organising principle – all of the content included and discussed should focus on answering this question.
Preparing a first draft
Before writing the article, it is useful to highlight several journals that you could submit the final article to. It also helps to familiarise yourself with these journals’ styles, article structures and formatting instructions before starting to write. Many journals also have criteria that research articles should be able to satisfy. For example, all research article submissions to Clinical Pharmacist must demonstrate innovative or novel results that are sustainable, reproducible and transferable[10]
.
Having researched potential target journals, you should have a clear idea about your target audience, enabling you to pitch the level of the article appropriately[11]
(see ‘Box 1: Top tips to prepare for writing’).
Box 1: Top tips to prepare for writing
- Know the focus of the paper – identify two or three important findings and make these the central theme of the article;
- Gather important data, perform any analyses and make rough data plots and tables beforehand. These can then be refined for inclusion or submitted as supplementary information if needed;
- Organise your results to flow in a logical sequence;
- Know the structure and requirements of your target journals (check websites and author guidelines, as well as published articles);
- Think about the style of the piece and look to pitch the article at the level of the intended audience;
- Clarity should be your guiding principle.
Structuring a research article
Most research articles follow a similar structure and format that includes an abstract, introduction, methods, results and discussion, as well as a summary of the key points discussed in the article.
One approach is to start with the methods section, which can be derived from the protocol and any pilot phase. Many of the figures and tables can be constructed in advance, which will help with writing the results section. The questions addressed by the study can be used alongside the results to formulate the introduction, which can guide how the discussion is written[11]
.
Clinical Pharmacist, like other peer-reviewed journals, has specific author guidelines and formatting instructions to help authors prepare their articles[10]
,[12]
,[13]
. The following sections will discuss the required sections and important considerations for authors when writing.
Title, abstract and keywords
The title, abstract and keywords are essential to the successful communication of research. Most electronic search engines, databases (e.g. PubMed/MEDLINE) and journal websites extract words from them to determine whether your article will be displayed to interested readers[14]
,[15]
,[16]
,[17]
, enabling accurate dissemination and leading to future citations.
In addition, the title and abstract are usually freely available online. If an article is not published in an ‘open access’ format, (i.e. it is free and immediately available online and access is combined with the rights to use these articles fully in the digital environment)[18]
, or if the reader does not have a subscription to the journal, they will have to decide on whether to pay to access the full article to continue reading. Therefore, it is imperative that they are informative and accurate.
The title should accurately reflect the research, identify the main issue and begin with the subject matter, while being both simple and enticing enough to attract the audience[19]
. Authors should avoid using ‘a study of’, ‘investigations into’ and ‘observations on’ in titles. It is also worth remembering that abstracting and indexing services, such as MEDLINE, require accurate titles, because they extract keywords from them for cross-referencing[19]
.
Many journals require the abstract to be structured in the same way as the main headings of the paper (e.g. introduction, methods, results, discussion and conclusion) and to be around 150–300 words in length[10]
. In general, references should not be cited in the abstract.
Introduction
The introduction should provide the background and context to the study. Two or three paragraphs can be dedicated to the discussion of any previous work and identification of gaps in current knowledge. The rest of the introduction should then outline what this piece of work aims to address and why this is important, before stating the objectives of the study and the research question[20]
.
Methods
The methods section should provide the reader with enough detail for them to be able to reproduce the study if desired[3]
. The context and setting of the study should be described and the study design specified. The section should further describe the population (including the inclusion and exclusion criteria), sampling strategy and the interventions performed. The main study variables should be identified and the data collection procedures described[3]
.
Authors should provide specific, technical and detailed information in this section. Several checklists and guidelines are available for the reporting of specific types of studies:
- CONSORT is used for developing and reporting a randomised controlled trial[21]
; - The STARD checklist can help with designing a diagnostic accuracy study[22]
; - The PRISMA checklist can be used when performing a metaâ€analyses or systematic review, but can also help with compiling an introduction[23]
.
For the reporting of qualitative research studies, authors should explain which research tradition the study utilises and link the choice of methodological strategies with the research goals[24]
.
For studies describing the development of new initiatives or clinical services, authors should describe the situation before the initiative began, the establishment of priorities, formulation of objectives and strategies, mobilisation of resources, and processes used in the methods section[10]
.
Statistics
The final portion of the methods section will include the statistical methods used to analyse the data[25]
. The statistical methods employed should be described with enough detail to enable a knowledgeable reader with access to the original data to be able to judge its appropriateness for the study and verify the results[3]
. For survey-based studies and information on sampling frame, size and statistical powers, see ‘When to use a survey in pharmacy practice research’
[26]
.
Findings should be quantified and presented with appropriate indicators of measurement error or uncertainty (e.g. confidence intervals). Authors should avoid relying solely on statistical hypothesis testing, such as P values, because these fail to convey important information about effect size and precision of estimates[3]
. Statistical terms, abbreviations and most symbols should be defined, and the statistical software package and versions used should be specified. Authors should also take care to distinguish prespecified from exploratory analyses, including subgroup analyses[3]
.
Results
The results section should be straightforward and factual and all of the results that relate to the research question should be provided, with detail including simple counts and percentages[27]
. Data collection and recruitment should be commented on and the participants described. Secondary findings and the results of subgroup analyses can also be presented[27]
.
Figures, schemes and tables
To present data and results of the research study, figures, schemes and tables can be used. They should include significant digits, error bars and levels of statistical significance.
Tables should be presented with a summary title, followed by caption, a sentence or two that describes the content and impact of the data included in the table. All captions should provide enough detail so that the table or figure can be interpreted and understood as stand-alone material, separate from the article.
Figures should also be presented with a summary title, a caption that describes the significant result or interpretation that can be made from the figure, the number of repetitions within the experiment, as well as what the data point actually represents. All figures and tables should be cited in the manuscript text[11]
.
When compiling tables and figures, important statistics, such as the number of samples (n), the index of dispersion (standard deviation [SD], standard error of the mean [SEM]), and the index of central tendency (mean, median or mode), must be stated. The statistical analysis performed should also be included and specific statistical data should be indicated (e.g. P values)[11]
.
Discussion and conclusions
The discussion section should state the main findings of the study. The main results should be compared with reference to previous research and current knowledge, and where this has been extended it should be fully described[2]
,[11]
,[25]
. For clinical studies, relevant discussion of the implications the results may have on policy should be included[10]
. It is important to include an analysis of the strengths and limitations of the study and offer perspectives for future work[2]
. Excessive presentation of data and results without any discussion should be avoided and it is not necessary to cite a published work for each argument presented. Any conclusions should include the major findings, followed by a brief discussion of future perspectives and the application of this work to other disciplines[10]
.
References
The list of references should be appropriate; important statements presented as facts should be referenced, as well as the methods and instruments used. Reference lists for research articles, however, unlike comprehensive reviews of a topic, do not necessarily have to be exhaustive. References to unpublished work, to documents in the grey literature (technical reports), or to any source that the reader will have difficulty finding or understanding should be avoided[27]
. Most journals have reference limits and specific formatting requirements, so it is important to check the journal’s author guidelines[10]
,[11]
,[12]
,[13]
,[19]
.
Authorship and acknowledgements
Determining contributors who qualify as authors and those who should be acknowledged can be difficult. Clinical Pharmacist follows guidance from the International Committee of Medical Journal Editors, which recommends that authorship be based on the following four criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved[3]
.
Therefore, only individuals who meet all four criteria should be identified as authors[3]
. The contribution of individuals who do not meet all four criteria should instead be included in the acknowledgements.
In addition, a statement that recognises any funding sources for the work should be added to the acknowledgements. This statement should adhere to the guidelines provided by the funding institution[11]
.
Supplementary and supporting information
A key principle of research publication is that others should be able to replicate and build upon an author’s published claims. Therefore, submitted manuscripts should contain the necessary detail about the study and analytical design, and the data must be available for editors and peer-reviewers to allow full evaluation to take place. This is now commonplace and is seen as best practice. Author guidelines now include sections related to misconduct and falsification of data[28]
. By participating in self-archiving practices and providing full data sets, authors can play their part in transparency.
The Royal Pharmaceutical Society website hosts a database to help share data from research studies. The map of evidence collates existing evidence and ongoing initiatives that can ultimately inform policy and practice relating to pharmacy; enables the sharing and showcasing of good pharmacy practice and innovation; and aims to increase the knowledge exchange and learning in pharmacy and pharmaceutical sciences[29]
.
Revising your article prior to submission
Once a draft research article has been prepared, it should be shared among all of the co-authors for review and comments. A full revision of the draft should then take place to correct grammar and check flow and logic before journal submission. All authors will have to agree on the authenticity of the data and presentation before formal submission can take place[3]
(see ‘Box 2: Common mistakes and reasons why research articles are rejected for publication’).
Box 2: Common mistakes and reasons why research articles are rejected for publication
- Lack of novelty and importance of the research question;
- Poor study design;
- Methods not accurately and adequately described;
- Results poorly reported, along with little analysis of data;
- Lack of statistical analysis;
- Not acknowledging the study’s limitations;
- Providing unsupported conclusions or overstating the results of the study;
- Poor writing;
- Not following the journal’s style and formatting guidance;
- Submitting a manuscript that is incomplete or outside of the aims and scope.
Selecting a journal and submitting your manuscript
It is important to select a journal for submission wisely because this choice can determine the impact and dissemination of your work [13]
. Impact factor (a measure of the frequency with which the average article in a journal has been cited in a particular year), the scope and readership of a title may also influence your choice.
Furthermore, approval and adequate disclosures must be obtained from all co-authors. A conflict of interests form is also completed as part of the submissions process (normally completed by the lead author on behalf of all authors).
Many journals now request that a cover letter is also submitted to the editor, putting the study in context and explaining why the research is of importance to their audience and why it should be considered for publication in their journal.
Once this is all completed, the article can be formally submitted (usually via email or an online submission system). Figure 2 provides a sample process for a manuscript once submitted to a journal for consideration for publication.
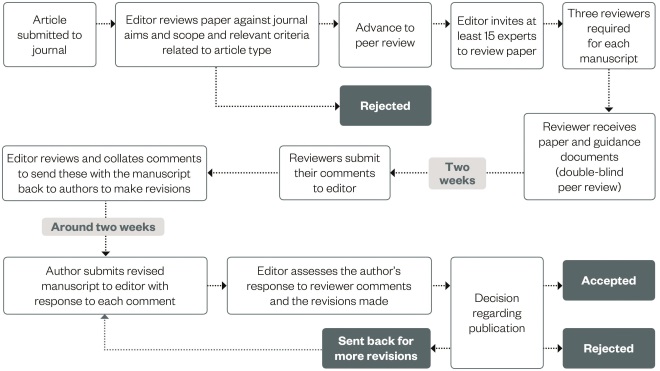
Figure 2: Sample process for a submitted manuscript
Source: The Pharmaceutical Journal
All journals follow a similar process for article submissions, whether they use a formal online submissions system or simply email. Clinical Pharmacist uses a process similar to this and it is useful for authors to be aware of how their submission may progress once submitted to a journal for publication.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Boyer E. Scholarship reconsidered: Priorities for the professoriate. 1990. Princeton, NJ: The Carnegie Foundation for the Advancement of Teaching.
[2] Hoogenboom BJ & Manske RC. How to write a scientific article. Int J Sports Phys Ther. 2012;7(5):512–517. PMCID: PMC3474301
[3] International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2014. Available at: http://www.icmje.org/icmje-recommendations.pdf (accessed November 2016).
[4] Dowdall M. How to be an effective peer reviewer. Clinical Pharmacist 2015;7(10). doi: 10.1211/CP.2015.20200006
[5] Nahata MC. Tips for writing and publishing an article. Ann Pharmaco. 2008;42:273–277. doi: 10.1345/aph.1K616
[6] Dixon N. Writing for publication: A guide for new authors. Int J Qual Health Care. 2001;13:417–421. doi: 10.1093/intqhc/13.5.417
[7] Keys CW. Revitalizing instruction in scientific genres: Connecting knowledge production with writing to learn in science. Sci Educ. 1999;83:115–130.
[8] Gopen G & Swan J. The science of scientific writing. Am Sci. 1990;78:550–558. Available at: http://www.americanscientist.org/issues/pub/the-science-of-scientific-writing (accessed November 2016)
[9] Hulley S, Cummings S, Browner W et al. Designing clinical research. 3rd ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2007.
[10] The Pharmaceutical Journal and Clinical Pharmacist. Author Guidance (2015). Available at: http://www.pharmaceutical-journal.com/for-authors-and-referees/article-types/#Practice_reports (accessed November 2016)
[11] Fisher JP, Jansen JA, Johnson PC et al. Guidelines for writing a research article for publication. Mary Ann Liebert Inc. Available at: https://www.liebertpub.com/media/pdf/English-Research-Article-Writing-Guide.pdf (accessed November 2016)
[12] Nature. Author Resources: How to write a paper. Available at: http://www.nature.com/authors/author_resources/how_write.html (accessed November 2016)
[13] Wiley Online Library. Resources for authors and reviewers: preparing your article. Available at: http://olabout.wiley.com/WileyCDA/Section/id-828006.html (accessed November 2016)
[14] SAGE Publications. Help readers find your article. Available at: http://www.uk.sagepub.com/journalgateway/findArticle.htm (accessed November 2016)
[15] Bem DJ. Writing the empirical journal article. In: MP Zanna & JM Darley (Eds.), The complete academic: a practical guide for the beginning social scientist (pp. 171–201). New York: Random House; 1987.
[16] Fathalla M & Fathalla M. A practical guide for health researchers. Available at: http://www.emro.who.int/dsaf/dsa237.pdf (accessed November 2016)
[17] Coghill A & Garson L (Eds.). Scientific Papers. In: A Coghill & L Garson (Eds.), The ACS Style Guide, 3rd Edition (pp. 20–21). New York: Oxford University Press, 2006.
[18] The Scholarly Publishing and Academic Resources Institute. Available at: http://sparcopen.org/open-access/ (accessed November 2016).
[19] Elsevier. Understanding the publishing process: how to publish in scholarly journals. Available at: https://www.elsevier.com/__data/assets/pdf_file/0003/91173/Brochure_UPP_April2015.pdf (accessed November 2016).
[20] SciDevNet. How do I write a scientific paper? 2008. Available at: http://www.scidev.net/global/publishing/practical-guide/how-do-i-write-a-scientific-paper-.html (accessed November 2016)
[21] Moher D, Schultz KR & Altman DG. CONSORT GROUP (Consolidatied Standards of Reporting Trials). The CONSORT statement: Revised recommendations for improving the quality of reports of parallelâ€group randomized controlled trials. Ann Intern Med. 2001;134:657–662. PMID: 11304106
[22] Bossuyt PM, Reitsma JB, Bruns DE et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Int Med 2003;138:40–44. PMID: 12513043
[23] Moher D, Liberati A, Tetzlaff J et al. The PRISMA Group (2009). Preferred reporting items for systematic reviews and metaâ€analyses: the PRISMA statement. PLoS Med 6(6):e1000097. doi: 10.1371/journal.pmed1000097
[24] Devers KJ & Frankel RM. Getting qualitative research published. Educ Health 2001;14:109–117. doi: 10.1080/13576280010021888
[25] Van Way CW. Writing a scientific paper. Nutr Clin Pract 2007;22:636–640. PMID: 18042951
[26] Kishore V. When to use a survey in pharmacy practice research. The Pharmaceutical Journal 296(7886). doi: 10.1211/PJ.2016.20200700
[27] Perneger PV & Hudelson PM. Writing a research article: advice to beginners. Int J Qual Health Care 2004;16(3):191–192. doi: 10.1093/intqhc/mzh053
[28] World Association of Medical Editors. Professionalism Code of Conduct. 2016. Available at: http://www.wame.org/News/Details/16 (accessed November 2016)
[29] Royal Pharmaceutical Society. Map of Evidence. Available at: http://www.rpharms.com/support/map-of-evidence.asp (accessed November 2016)