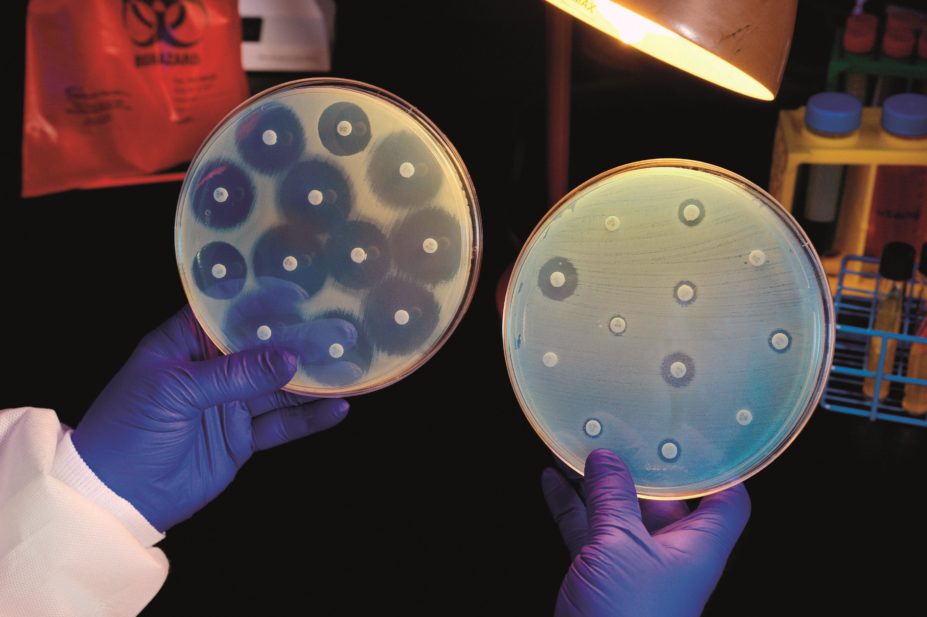
CDC / Science Photo Library
Summary box
In this article you will learn:
- The extent of antimicrobial resistance in Europe
- How antimicrobial resistance has developed in enterobacteria
- The steps you can take to improve antimicrobial stewardship
- How to get involved with European Antibiotic Awareness Day
The World Health Organization (WHO) estimates that the average human lifespan is extended by 20 years through use of antimicrobials[1]
. However, this is being threatened by increasing levels of antimicrobial resistance. Currently, antimicrobial resistance results in around 25,000 deaths and €1.5bn in healthcare expenses, lost productivity and associated costs across the European Union each year[2]
, and in 2013, England’s chief medical officer Sally Davies warned that we could start seeing deaths from sepsis developed following even minor surgery in as little as 20 years[3]
.
Resistance is increasing in all healthcare settings. A recent study[4]
analysed UK primary care data for almost 11 million prescriptions for antimicrobials used as monotherapy to treat upper and lower respiratory tract, otitis media and skin and soft tissue infections. It found that more than one in ten treatments were ineffective (indicated by a second prescription for antimicrobials within 30 days), and overall treatment failure rates increased by 12% between 1991 and 2012.
Recently, resistance has caused increased concerns in secondary care, with Public Health England (PHE) issuing guidance for acute trusts in 2014 following outbreaks of infections caused by carbapenemase-producing enterobacteria[5]
. Over the past few decades, resistance has increasingly developed in this family of bacteria against a range of antimicrobials (see Figure 1). Carbapenems were once considered last-line therapy, and resistance means new combinations of antimicrobials, such as intravenous tigecycline and colistin, are being used.
Figure 1. Evolution of enterobacteria resistance
Production of beta-lactamase by enterobacteria, which hydrolyses the beta lactam ring in many antimicrobials, has resulted in increased resistance rates. Some enterobacteria are now capable of producing carbapenemases that hydrolyse penicillins, cephalosporins, monobactams and carbapenems.
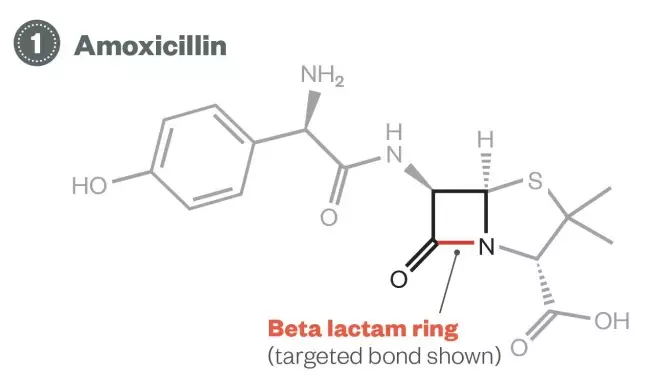
Evolution of enterobacteria resistance – First phase: amoxicillin
1) Amoxicillin: More than 60% of enterobacteria are now resistant to amoxicillin.
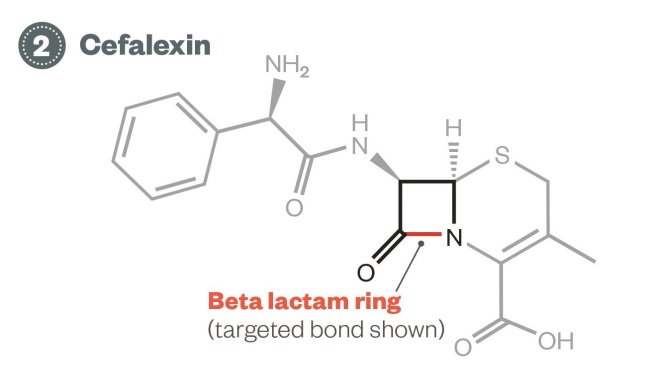
Evolution of enterobacteria resistance – Second phase: cefalexin
2) Cefalexin: Enterobacteria producing broad-spectrum beta-lactamases can inactivate first and second-generation cephalosporins, such as cefalexin.
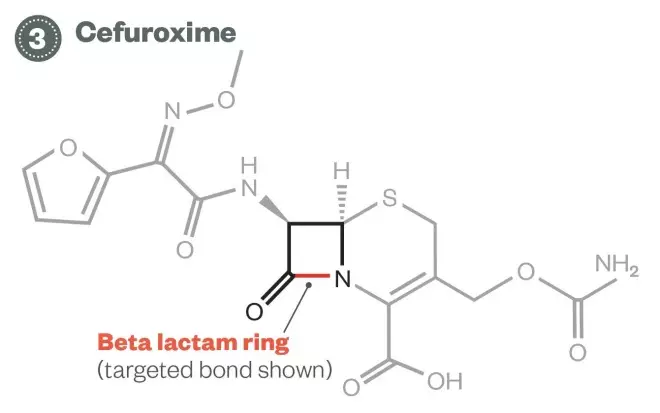
Evolution of enterobacteria resistance – Third phase: cefuroxime
3) Cefuroxime: Around 10% of enterobacteria in the UK now produce extended spectrum beta-lactamases, which can inactivate third-generation cephalosporins such as cefuroxime.
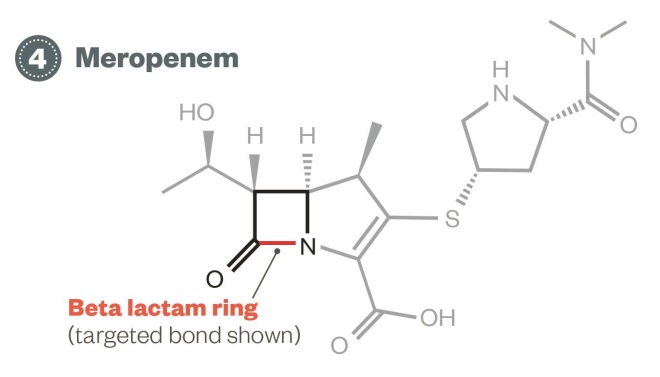
Evolution of enterobacteria resistance – Fourth phase: meropenem
4) Meropenem: Carbapenemase-producing enterobacteria strains are now being seen in the UK, resulting in resistance to antimicrobials such as meropenem that were are used to treat cephalosporin-resistant strains. Treatment options for enterobacteria resistant to meropenem are uncertain.
Since the publication of the UK five-year antimicrobial resistance strategy in 2013[6]
, there has been increased political recognition of the impact that antimicrobial resistance could potentially have on health systems around the world, and in April 2014, a WHO report[6]
further highlighted the high levels of antimicrobial resistance across member countries.
How does resistance occur?
Antimicrobial resistance follows the principles of Darwinian selection: any living organism placed in a hostile environment will, through a process of random mutation over multiple generations, develop mechanisms to increase their chances of survival.
With regard to antimicrobials, if a population of bacteria is placed in an antibiotic-rich environment, random bacterial mutations that prevent the antibiotic from reaching and attacking the target bacterial enzyme or organelle will confer a greater survival benefit. Bacteria with these mutations are therefore less likely to be killed, and will have an improved chance to multiply and determine the dominant characteristics of that population of bacteria.
Reducing resistance
National strategies have already been adopted to reduce antimicrobial resistance. In 2013, the UK government published a five-year strategy for reducing antimicrobial resistance[7]
. This strategy had three overarching aims:
1) improve the knowledge and understanding of antimicrobial resistance
2) conserve and steward the effectiveness of existing treatments
3) stimulate the development of new antimicrobials, diagnostics and novel therapies.
In September 2014, a similar strategy was adopted in the United States following a presidential executive order[8]
.
Pharmacists in all sectors can support the reduction in antimicrobial resistance by ensuring antimicrobials are only used when they are needed, the right choice of antimicrobial is made, and it is supplied and administered correctly.
European Antibiotic Awareness Day
For the past five years, the European Centre for Disease Prevention and Control has marked 18 November as European Antibiotic Awareness Day (EAAD).
This year, one of the focuses, promoted by the Department of Health, is for healthcare professionals and the public to sign up as an ‘Antibiotic Guardian’ and pledge to use antibiotics responsibly.
The following are suggested pledges for pharmacy teams:
- I will check that antibiotic prescriptions comply with local guidance and query those that do not.
- The next time a customer presents with a self-limiting infection (e.g. coughs or colds), I will use the patient information leaflet to explain the potential duration of illness and how to treat their symptoms.
- I will undertake CPD activities to support my knowledge of antimicrobial use.
- When handing out a prescription that includes antimicrobials, I will ensure the patient: u
nderstands why they are taking the antimicrobial prescribed; i
s not allergic to the antimicrobial prescribed; k
nows how to take the antimicrobial, including the importance of completing the prescribed course; u
nderstands they should not share or reuse their antimicrobial; h
as been provided advice on how to manage infections; and h
as been offered a flu vaccine (if applicable).
Hospital pharmacists are already engaged in near-patient clinical roles that allow them to intervene in the event of inappropriate antimicrobial use. A lot of work on improving antimicrobial use in hospitals has focused on reducing healthcare-acquired infections such as Clostridium difficile and meticillin-resistant Staphylococcus aureus (MRSA), and these structures and processes can be applied throughout the organisation to improve overall antimicrobial use.
Pharmacists working as part of multidisciplinary clinical teams must ensure that where antimicrobials are used in their clinical area, the choice of agent and treatment plan complies with national standards and local microbiology expertise.
All hospitals should have an antimicrobial lead pharmacist who promotes antimicrobial stewardship based on the ‘Start Smart then Focus’ toolkit, developed by Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection (ARHAI)[9]
. This pharmacist should work closely with other infectious disease professionals, such as the local director of infection prevention and control, infection prevention nurses, medical microbiologists and consultants in infectious disease.
Community pharmacists should ask patients the reason antimicrobials have been prescribed when receiving prescriptions from primary care prescribers to ensure that antimicrobial treatment is appropriate.
Patients prescribed long-term antimicrobials can also be targeted for interventions, such as medicines use reviews. For many conditions (for example, lymphoedema and diabetic foot ulcers) long-term therapy may be appropriate, but the patient should be asked whether they know about the plan for ongoing management. Pharmacists should consider if the patient has been told how long they will be taking the antimicrobial, and potentially contact the prescriber to see if further tests have been arranged to monitor whether the antimicrobial is working, and what steps are being taken to review its use.
Community pharmacists and their teams can educate the public on antimicrobial use, either directly or by promoting resources such as the Treat Yourself Better website, developed by Pharmacy Voice and the Proprietary Association of Great Britain[10]
. In addition, pharmacy teams should ensure medicine counter assistants understand when antibiotics should be used, and when to recommend over-the-counter products to relieve symptoms.
Pharmacists can also work with local GPs, or contacts in the primary care organisation or commissioning body, to improve prescribing habits. For example, delayed prescriptions can be used to provide patients with an option to initiate antimicrobials if an infection gets worse.
There is also a need for community pharmacies to contribute to research on antimicrobial use and prescribing practices in UK community practice.
Primary care pharmacists can support prescribers by developing local guidelines based upon PHE’s guidance on diagnosing and managing infections in the community
[11]
. This document gives clear guidance as to which antimicrobial should be prescribed for which conditions.
Pharmacists involved in education and training should instil good prescribing practices and knowledge of antimicrobials at an early stage in the professional development of all healthcare professionals. In 2013, ARHAI and PHE published antimicrobial prescribing and stewardship competencies
[12]
, which summarise areas where training is required, including infection control, resistance and antimicrobial prescribing, and antimicrobial stewardship. This document can also be used by pharmacist prescribers to improve their practice and identify learning needs.
Kevin Frost is the antimicrobial lead pharmacist at Airedale NHS Foundation Trust.
References
[1] World Health Organization. Self-prescription of antibiotics boosts superbugs epidemic in the European Region. Copenhagen: WHO 2012.
[2] European Union Public Health. Antimicrobial Resistance [Online]. Available at: http://ec.europa.eu/health/antimicrobial_resistance/policy/index_en.htm (accessed 29 September 2014).
[3] Department of Health. Chief Medical Officer annual report 2011 volume 2. London:DH 2013.
[4] Currie CJ, Berni E, Jenkins-Jones S et al. Antibiotic treatment failure in four common infections in UK primary care 1991-2012: longitudinal analysis. BMJ 2014;349:g5493.
[5] Public Health England. Carbapenemase-producing Enterobacteriaceae: early detection, management and control toolkit for acute trusts. London: PHE 2014.
[6] World Health Organisation. Antimicrobial resistance: global report on surveillance. Geneva: WHO 2014.
[7] Department of Health. UK Five-year Antimicrobial Resistance Strategy 2013–2018. London: DH 2013.
[8] White House. Executive Order — Combating Antibiotic-Resistant Bacteria. Available from: http://www.whitehouse.gov/the-press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria (accessed 4 November 2014).
[9] Department of Health. Start smart then Focus. London: DH 2011.
[10] Treat Yourself Better. Available from: http://www.treatyourselfbetter.co.uk (accessed 4 November 2014).
[11] Public Health England. Primary care guidance: diagnosing and managing infections. London: PHE 2013.
[12] Department of Health and Public Health England. Antimicrobial prescribing and stewardship competencies. London: DH 2013.
You might also be interested in…

Everything you need to know about mpox
Norovirus and strategies for infection control
