Prof. P Motta / Dept of Anatomy / University "La Sapienza", Roma / Science Photo Library
In this article you will learn:
- The side effects associated with high-dose inhaled corticosteroids
- Which patients require an inhaled corticosteroid card
- Advice for patients starting inhaled corticosteroid therapy
Inhaled corticosteroids (ICSs) are indicated in the management of most patients with asthma and some patients with chronic obstructive pulmonary disease (COPD)
[1],[2]
.
NHS data for 2013–2014 suggests that, in England, of the 242 million ICS-containing inhalers dispensed at a cost of almost £355m, 39% were for high-dose inhalers.
A high dose of ICS is defined as ≥1,000mcg beclometasone dipropionate (BDP) equivalent per day. Fluticasone propionate, mometasone and the newer ultrafine particle BDP hydrofluoroalkane (HFA) inhalers (i.e. QVAR and Fostair) are considered twice as potent as standard BDP inhalers. However, dose equivalents are approximate, and the dose delivered will depend on other factors such as inhaler technique.
Place in therapy
The British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) and Global Initiative for Asthma (GINA) guidelines recommend the use of ICSs to reduce symptoms, improve lung function, reduce the frequency of severe exacerbations, including hospital and ICU admissions, and decrease the risk of mortality[3]
. For more information, see ‘Asthma: management’ in Clinical Pharmacist (November 2014), available at www.pharmaceutical-journal.com.
Patients with asthma who do not use an ICS regularly have poorer outcomes. A national review of asthma deaths in the UK in 2014 found that patients with asthma who did not use an ICS were at significantly greater risk of death[4]
.
In contrast, the place of an ICS in COPD is more uncertain. A Cochrane review[5]
did not find a clinically meaningful benefit of ICS use in terms of preventing exacerbations, improving lung function or health-related quality of life in patients with stable COPD. A clinical review of the risk-to-benefit ratio of ICSs in patients with COPD[6]
concluded that their use (particularly at high dose) in a typically older cohort, frequently with co-morbidities, put COPD patients at increased risk of ICS-associated side-effects. A further Cochrane review in 2012 warned that their use should be ‘weighed up against their significant side-effect profile’[7]
.
The reason for this difference in efficacy may be related to the mechanism of action of ICS. In asthma, inflammation is primarily caused by eosinophils, while in stable COPD neutrophils are predominant[8]
. Corticosteroids are more effective in reducing eosinophilic inflammation, which may explain this difference in clinical response.
Initiation
Patients who require prolonged high-dose ICS are at risk of systemic side effects, particularly immunosuppression and adrenal suppression, and should be issued with a corticosteroid treatment card[9]
(see ‘Doses of ICS that require a corticosteroid card in adults’). An ICS-specific card for patients, alongside guidance on counselling points for health professionals, has been developed by the London Respiratory Network and is available to download at www.pharmaceutical-journal.com.
Patients who are using high-dose ICS should be advised to inform the healthcare team responsible for their treatment if they fall ill for any reason, as this may affect the dose required. In addition, they should see a GP if they have symptoms that could be related to ICS treatment (e.g. worsening fatigue, muscle weakness, loss of appetite, unintentional weight loss, dizziness, unexplained nausea, vomiting and diarrhoea).
All patients taking ICS who have never had chickenpox should be advised to avoid people with chickenpox or shingles, and to see a doctor if they come in contact with someone with either illness and then feel unwell.
Inhaled corticosteroids do not usually interact with other medicines. However, they are metabolised by the cytochrome P450 isoenzyme CYP3A4, and some medicines (e.g. telaprevir, ritonavir and itraconazole) may increase plasma concentrations of inhaled budesonide and fluticasone, which increases the patient’s risk of adrenal suppression.
Patients who smoke may require higher doses of ICS compared with non-smokers for the same therapeutic effect[10]
. It is therefore important that all smokers using an ICS should be offered help to stop smoking, as this may reduce the dose required by the patient and minimise the risk of side effects.
Treatment with high-dose ICS can result in clinically significant suppression of endogenous cortisol. A systematic review and meta-analysis of 13 studies[11]
evaluated the effect of several inhaled and oral corticosteroids in patients’ 8am cortisol level and established similar suppression rates between inhaled fluticasone propionate and oral prednisolone.
The analysis suggested 1,000mcg of inhaled fluticasone propionate was approximately equivalent to 10mg oral prednisolone and at this dose, half of the patients were sufficiently suppressed to be unable to mount the necessary adrenal response to stress. Therefore all patients taking an oral corticosteroid for more than three weeks or ‘prolonged high dose inhaled steroids’ should have the dose tapered gradually.
Doses of ICS that require a corticosteroid card in adults
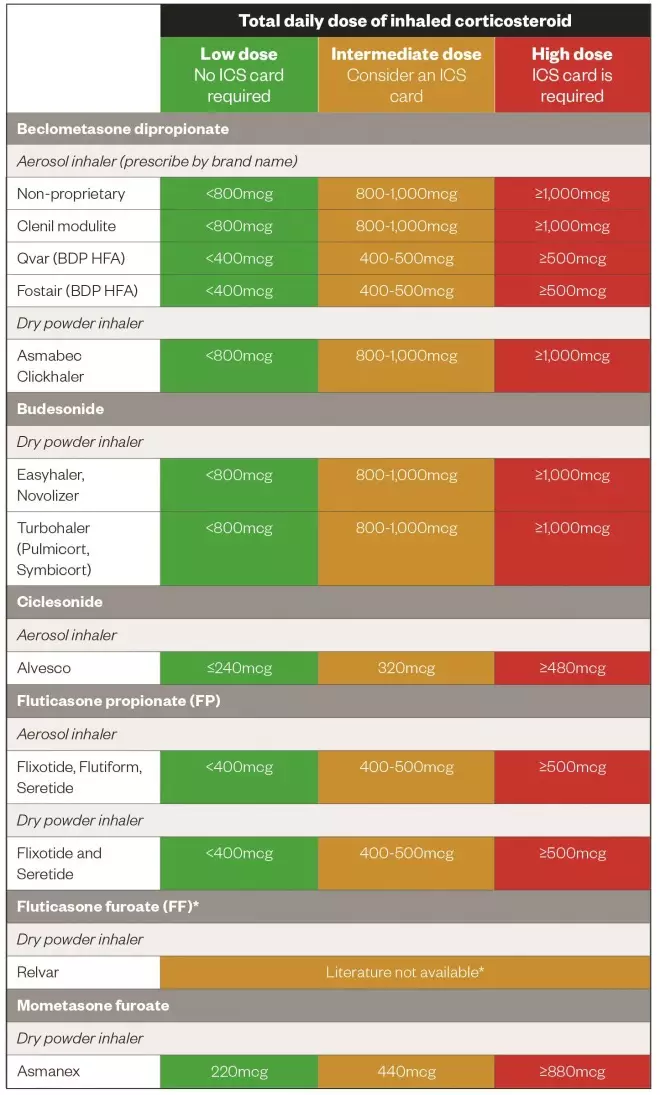
*Fluticasone furoate 92mcg once daily is approximately equivalent to fluticasone propionate 250mcg twice daily. This could be interpreted as being equivalent to 1,000mcg of beclomethasone dipropionate, but caution is advised as direct comparator studies have not been published.
- Dosage equivalents are approximate and dose delivered will depend on other factors such as inhaler technique
- Encourage patients to use appropriate breathing techniques according to inhaler device e.g.: ’slow and steady’ for an aerosol inhaler, “quick and deep” for a dry powder inhaler
- If a patient is using nasal corticosteroids and an ICS, they should be assessed individually. For example,for a patient taking nasal corticosteroids and 800–1,000mcg of BDP equivalent/day, a corticosteroid safety card is recommended.
- Before prescribing, patients should always have their therapy reviewed for continued appropriateness and if necessary, issued an ICS card.
High-dose ICS safety card

Side effects
Local ICS side effects can be distressing and may affect adherence to treatment. They include a sore throat, hoarse voice and opportunistic oral candidiasis infection.
All patients using ICS should be advised to rinse out their mouth with water (spitting out the rinse) and brush their teeth after using their device, which will reduce the risk of developing a sore throat or hoarseness. Spacer devices can also be used to reduce oropharyngeal deposition of drug particles, and should be recommended for all ICS multi-dose inhalers[12]
.
Patients using ICS who present with white patches (plaques) in their mouth should be referred to their GP, as this could be oral candidiasis and will require treatment with a topical antifungal (e.g. nystatin liquid or amphotericin lozenges), or may be a sign of a more serious condition.
Systemic side effects may be dose-related, or more pronounced in patients with co-morbidities (e.g. diabetes, osteopenia) or COPD. A dose-related increase in the risk of developing type 2 diabetes and its subsequent complications has been reported[13]
, and data have shown an association between high-dose ICS and tuberculosis[14]
.
Patients with COPD are at a higher risk of developing pneumonia than people who do not have COPD[15]
, and this risk appears to be further amplified in patients using ICS[16]
, particularly at high doses. A Cochrane review was unable to determine a statistically significant difference in incidence of pneumonia, mortality or serious adverse events between patients prescribed fluticasone propionate, budesonide or BDP[17]
. All patients with COPD should be offered a pneumococcal vaccination and the annual influenza vaccination.
High-dose ICS may increase the risk of fractures in COPD[18]
. However, the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) asthma guidelines advise bone mineral density monitoring and treatment only for adults on long-term or frequent courses of corticosteroid tablets, and not those on ICS.
Dose optimisation
The main strategy to minimise the risk of ICS-induced side effects is dose optimisation. In most patients with asthma, there is limited evidence that increasing the dose of ICS above 800mcg BDP equivalent per day improves asthma control, although high doses are associated with an increased risk of adverse events[19]
.
The BTS/SIGN guidelines recommend that the dose of any ICS should be reduced by 25–50% in patients with good asthma control (i.e. no exacerbations for three months), to the lowest dose that controls symptoms[2]
. It is unlikely a patient with asthma would have their ICS completely withdrawn.
Before increasing the dose of an ICS, it is important to check the patient’s adherence to therapy, and to improve ICS delivery to the lungs. This can be done by optimising the patient’s inhaler technique, or by using a metered dose inhaler (MDI) with a spacer device, which can improve lung deposition.
Grainne d’Ancona is a Principal Pharmacist at Guy’s and St Thomas’ NHS Foundation Trust.
References
[1] National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: NICE 2010.
[2] British Thoracic Society and Scottish Intecollegiate Guidelines Network. British Guideline on the Management of Asthma: A national clinical guideline. London: BTS 2014.
[3] Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2014.
[4] Royal College of Physicians (London). National Review of Asthma Deaths 2014. London: RCP 2014.
[5] Yang I, Fong K, Sim E et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease (Review). Cochrane Datab Sys Rev 2007.
[6] Price D, Yawn B, Brusselle G et al. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Resp J. 2013;22(1):92–100.
[7] Nannini LJ, Lasserson TJ & Poole P. Combined corticosteroid and long-acting beta (2)-agonist in one inhaler versus long-acting beta (2)-agonists for chronic obstructive pulmonary disease. Cochrane Datab Sys Rev 2012.
[8] Barnes PJ. Similarities and differences in inflammatory mechanisms of asthma and COPD. Breathe 2011;7(3):229–238.
[9] Commission on Human Medicines and Medicines and Healthcare Products Regulatory Agency. Current Problems in Pharmacovigilance Volume 31: 2006.
[10] Tomlinson JEM, McMahon AD, Chaudhuri R et al. Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax 2005;60(4):282–287.
[11] Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med 1999;159(9):941–955.
[12] Singh D, Collarini S, Poli G et al. Effect of AeroChamber Plus on the lung and systemic bioavailability of beclometasone dipropionate/formoterol pMDI. Br J Clin Pharmacol 2011;72(6):932–939.
[13] Suissa S, Kezouh A & Ernst P. Inhaled Corticosteroids and the Risks of Diabetes Onset and Progression. Am J Med 2010;123(11):1001–1006.
[14] Kim JH, Park JS, Kim KH et al. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest 2013;143(4):1018–1024.
[15] Farr BM, Woodhead MA, Macfarlane JT et al. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med 2000;94(5):422–427.
[16] Calverley PM, Anderson JA, Celli B et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New Engl J Med 2007;356(8):775–789.
[17] Kew KM & Seniukovich A. Do inhaled steroids increase the risk of pneumonia in people with chronic obstructive pulmonary disease (COPD)? Cochrane Datab Sys Rev 2014.
[18] Loke YK, Cavallazzi R & Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax 2011;66(8):699–708.
[19] Masoli M, Holt S, Weatherall M et al. The dose-response relationship of inhaled corticosteroids in asthma. Curr Allergy Asthma Rep 2004;4(2):144–148.