
Borthwick ISTOCKPHOTO.COMPharmacists
This content was published in 2008. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Safe and effective prescribing of intravenous fluids requires understanding of the physiology of fluid and electrolyte homeostasis, physiological responses to injury and disease, as well as knowledge of the properties of intravenous fluids. Research has shown that the prescribing of intravenous fluids is generally left to junior doctors — whose knowledge may be limited.1 Iatrogenic problems arising from inappropriate fluid therapy can increase morbidity and prolong hospital stays. Pharmacists should be prepared to advise on the prescribing of IV fluids alongside that of other medicines.
Basic fluid physiology
Fluid and electrolyte levels in the body are kept relatively constant by several homeostatic mechanisms. Normally, fluid is gained from a person’s food and drink intake (including a small amount from carbohydrate metabolism). It is lost via the urine, sweat and faeces, as well as through insensible losses via the lungs and skin. Within the body, water is distributed into intracellular and extracellular compartments. The extracellular compartment comprises both interstitial and plasma compartments. Water moves freely across the membranes that separate the compartments to maintain osmotic equilibrium.
Sodium-potassium pumps on cell membranes normally ensure that potassium is pumped into cells and sodium is pumped out, thus the intracellular sodium concentration is lower than the extracellular sodium concentration (the reverse applies to potassium) — see Panel 1.
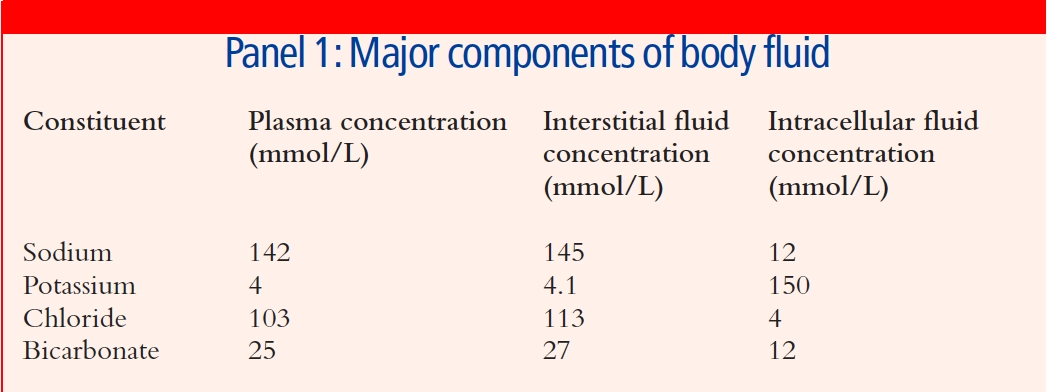
In healthy individuals, volume homeostasis is regulated largely by antidiuretic hormone (ADH). Osmoreceptors and baroceptors detect small decreases in osmolality and blood pressure, triggering the release of ADH. This elicits a sensation of thirst and reduces renal excretion of water. Renal mechanisms also play a part in volume homeostasis — the renin-angiotensin mechanism is activated by falling renal perfusion pressure.
It is important to remember that normal homeostatic mechanisms may not work well after injury (due to trauma or surgery), or during episodes of sepsis or other critical illness.
IV fluid therapy indications
IV fluid therapy is used to maintain homeostasis when enteral intake is insufficient (eg, when a patient is “nil by mouth” or has reduced absorption), and to replace any additional losses. These losses may occur from the gastrointestinal tract (due to vomiting, diarrhoea or a fistula) or the urinary tract (eg, diabetes insipidus), or be caused by blood loss from trauma or surgery. In addition, insensible losses can increase during fever or after suffering from burns because the barrier function of the skin is impaired.
Fluids can accumulate into spaces that normally contain minimal fluid volumes (eg, the peritoneal or pleural cavities) during surgery, anaesthesia or as a result of inflammatory conditions (eg, sepsis). This is known as “third spacing” and is caused by vasodilation and “leakage” of vascular epithelial walls. This breakdown of normal compartment integrity can result in loss of circulating intravascular volume.
Assessing requirements
Patients’ medical histories give an indication of their expected fluid status. Causes of dehydration include preoperative fasting, ongoing gastrointestinal illness and self-neglect following acute confusion. Knowing a detailed diagnosis is vital to gain information on the likely composition of the fluid lost. Practitioners also need to be aware of any concurrent conditions that can alter fluid distribution or make patients more susceptible to adverse effects from fluid therapy (eg, a history of heart failure).

Identifying dehydration
On physical examination, signs of dehydration include:
- Thirst
- Reduced skin turgor (elasticity)
- Dry mucous membranes
- Increased capillary refill time
- Altered level of consciousness
If a patient is suffering from fluid (volume) depletion, then his or her heart rate will increase to improve cardiac output and raise blood pressure, hereby maintaining tissue oxygenation. Blood pressure only falls after the intravascular volume has dropped by 20–30 per cent.
Urine becomes concentrated in cases of volume depletion — more severe cases result in a fall in urine output. Raised plasmaurea (above 6mmol/L) and sodium levels (above 145mmol/L) can indicate dehydration, as can acidosis on a blood gas analysis.
Signs and symptoms need to be evaluated as a whole, since their specificity in isolation is limited. It should be borne in mind that co-existing conditions may alter results (eg, tachycardia may be suppressed by concurrent drug therapy).
Fluid balance
An accurately monitored fluid balance of overall intake and output is vital to tailor fluid administration. Losses via urine, drains, stoma or nasogastric aspirates should be documented. In addition, insensible losses via the respiratory tract and skin (adjusted for body temperature) should be estimated and compared with patients’ normal physiological requirements. It is important to interpret all observations in the context of a patient’s clinical diagnosis — an oedematous patient may show a positive fluid balance but still be intravascularly depleted, resulting in insufficient tissue perfusion and oxygenation.
Special considerations
Some pathological conditions require special consideration. Patients with major burns require copious amounts of intravenous fluids, calculated according to body weight and percentage of body surface area affected. In traumatic brain injury, fluid volume may be adjusted according to mean arterial pressure because this is related to cerebral perfusion pressure. Large amounts of IV fluids are also often required following trauma or septic peritonitis. Fluid administration has to be particularly carefully balanced in individuals with heart failure, renal impairment or apparent respiratory failure.
Measuring success
Similarly to any drug treatment, IV fluid administration requires monitoring for clinical response and adverse effects to ensure its safety and efficacy.
While dehydration will lead to malperfusion, kidney failure and eventually cell death, excessive administration of fluid is also associated with complications. Various studies have shown that outcomes for post-surgical and critical care patients can be improved with targeted, and even restrictive, fluid therapy — rather than administering fluids according to a set recipe of millilitres per kilogram of body weight. In this context, “restrictive” therapy should not be misunderstood as administering less fluid than required, but not administering anymore than necessary.
There are several ways to evaluate fluid therapy. Successful therapy may be indicated by:
- Clinical signs (eg, improved urine output, reduced capillary refill time and reduced heart rate)
- Biochemical signs (eg, normalisation of sodium, urea and creatinine levels)
- Patients’ subjective experiences (eg, they “feel better” or are no longer thirsty)
These findings may be absent if masked by other factors. For example, urine output can remain low for 24 hours after surgery, as part of the normal response to injury, even in patients receiving adequate fluid input.
Urine output can also be affected by diuretics that are started inappropriately to maintain urine output, without knowledge of the patient’s fluid status.
Daily weighing is the simplest and most reliable means of monitoring fluid status, but it does not provide any information about the distribution of administered fluids. Invasive techniques can provide a more detailed picture of the intravascular volume status.
Invasive techniques
The measurement of central venous pressure (CVP) via a central venous catheter is often used to assess intravascular volume. Absolute values will be influenced by several patient-specific parameters, but the trend of CVP in response to a “fluid challenge” (see Panel 2) is a good indication of whether a patient’s fluid volume is increasing. The use of this technique has recently come under question,2 but it remains common in routine practice.
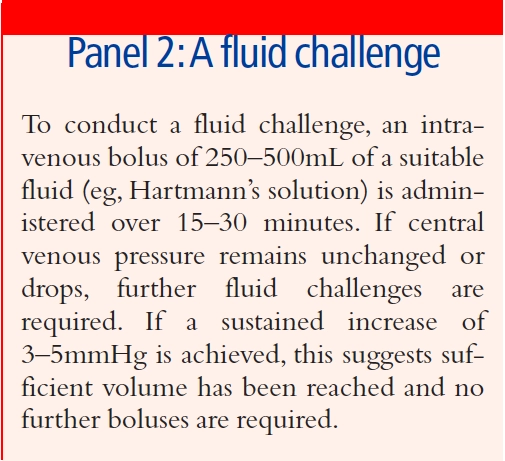
Similar results can be derived from cardiac output measurements, using a variety of techniques such as oesophageal Doppler or thermodilution. These methods will be restricted to use in critical care areas and, since values are influenced by several parameters (eg, the use of vasoactive medicines), expert knowledge is required for their interpretation and application to clinical treatment.
Timing
Timing of fluid therapy can sometimes be more important than volume administered. It has been shown that by treating critically ill patients who require fluid resuscitation aggressively and early (giving them most of their resuscitation fluids within six hours of their deterioration), they have better outcomes than those patients whose fluid resuscitation is delayed (when most of it is administered more than six hours after they deteriorate).2,3
Types of fluid available
IV fluids can be categorised according to their physical composition:
- Crystalloids are solutions of small molecules in water (eg, sodium chloride, glucose, Hartmann’s)
- Colloids are dispersions of large organic molecules (eg, Gelofusin, Voluven)
Fluids can also be categorised according to their mechanism of distribution in the body or their electrolyte loads.
The different types of fluid distribute into the various fluid compartments in different ways (see Figure 1). In general, colloids remain in the intravascular space, while crystalloids distribute more readily into other tissues. Sodium chloride (NaCl) distributes into the extracellular space (intravascular and interstitial spaces). Glucose solutions distribute throughout the intravascular, interstitial and intracellular compartments.
Glucose solution, at a concentration of 5 per cent, has the same tonicity as plasma and is used for fluid therapy. Hypertonic solutions of glucose (10 per cent or 50 per cent) are used when glucose substitution is required (eg, to treat hypoglycaemia).
Hypo- and hypertonic solutions of NaClare also available, but their use is limited. Hypotonic NaCl is used to treat hypernatraemia. Hypertonic NaCl is sometimes used to correct hyponatraemia, and very strong solutions are used to manage aspects of head injury. Careful monitoring is required for these uses.
Colloid solutions
The characteristics of colloid infusions depend mainly on their molecular size. Many modern colloid solutions are based on hydroxyethyl starches (HESs) which have high molecular weights (70,000 –450,000 daltons) and can provide volume expansion for 6–24 hours. The solutions’ duration of action depends on its starch’s molecular size (larger molecules tend to have a longer duration), the rate of degradation and the permeability of the vascular endothelium.
Tetrastarch (40 per cent substituted HES), with a mean molecular weight of 130,000 daltons, exerts its effect for 4–6 hours. Modified fluid gelatine, which is derived from animal collagen, has a molecular weight of 30,000 daltons. Its effective half life is about four hours, but its volume-restoring effect may be shorter in patients with capillary leakage.
Choosing a fluid
Deciding which fluids are appropriate for each patient depends on the type of fluid that has been lost and the body compartment(s) that require additional volume. Patients’ renal function, cardiac function, blood gases and electrolyte levels also need to be considered, where available.
For a patient requiring fluid maintenance who has healthy kidneys and no comorbidities that affect fluid homeostasis, a suitable regimen will be a combination of a glucose-based IV fluid and a second fluid to boost intravascular volume (usually a sodium-based fluid). The latter will need to provide 1–1.5mmol/kg sodium and 1mmol/kg potassium per day. Calcium and magnesium supplementation should be considered if oral intake is interrupted for more than a few days and should be guided by plasma level measurements.
Often this will be provided as a combination of NaCl 0.9 per cent and glucose 5 per cent infusions, or as “dextrose-saline” (usually 2.5–3L of a combined infusion of glucose 4 per cent and NaCl 0.18 per cent over 24 hours).
This dextrose-saline solution is not recommended for long-term maintenance because it provides less than the required daily amount of sodium, unless excess volume is administered. Also, it is only slightly more efficient than plain glucose infusions at restoring intravascular volume, so the required extra volume would increase the risk of interstitial oedema.
Fluid resuscitation
Fluid resuscitation is required in situations where there is acute circulatory shock or intravascular volume depletion. The objective is to restore circulating volume and increase cardiac output, thereby restoring tissue perfusion and oxygen delivery.
In resuscitation situations, restoring intravascular volume is initially important, and any sodium or colloid-based fluid can be used to do this. Fluids that distribute throughout total body water (eg, glucose) do not restore intravascular volume and can exacerbate interstitial oedema in patients who are septic or suffering from other inflammatory conditions. Practitioners should remember that any fluid (and its associated electrolyte load) administered during the resuscitation phase will have to be cleared or redistributed by the body. This may take several days or weeks in a patient with impaired homoeostasis.
Considering the complications associated with excessive NaCl load (see below), a crystalloid of a more “physiological” composition (eg, Hartmann’s solution) is preferred if large volumes of fluid are required.
Colloids vs crystalloids
Colloids allow fast restoration of intravascular volume, but there has been much debate about their safety and superiority over crystalloids. A recently updated Cochrane meta-analysis4 showed no difference in mortality between patients treated with colloids and those treated with crystalloids for fluid resuscitation. In the original Cochrane review, there was particular controversy regarding albumin infusions. Subsequently, the SAFE study,5 which compared albumin and saline for fluid resuscitation, demonstrated no difference in outcomes between albumin 4 per cent and NaCl 0.9 per cent for patients in intensive care.
With colloid infusions being significantly more expensive than crystalloid infusions, they are often less cost-effective. The use of albumin is now restricted in the UK to conditions where the synthesis of clotting factors is reduced (eg, severe hepatic failure). However, it is not in other countries (eg, Australia, New Zealand).
The lower overall volume load with colloids is often pointed out as an advantage. Regarding their ability to replenish the intravascular volume, 3L of a crystalloid solution is generally assumed to be equivalent to 1L of colloid solution. However, the SAFE study reported a ratio of 1.4L of normal saline to 1L of albumin.
Gelatin infusions have a similar molecular size to albumin and, therefore, may not allow a significant reduction in administered volume either. It may be possible to use smaller volumes of solutions of large starch molecules (eg, Voluven) to replenish intravascular volume. In particular, in conditions where there is increased epithelial wall permeability (eg, sepsis, other inflammatory conditions, anaesthesia), starches may be more effective at reducing leakage into the interstitial space by increasing oncotic pressure.
A 2007 meta-analysis6 failed to show any difference in outcome between different types of colloids. However, a wide variety of studies were included and further research is required. Colloids are associated with their own adverse effect profiles, which should betaken into account when making choices for individual patients.
Until recently, all colloids contained considerable amounts of sodium, so their administration invariably resulted in patients receiving a substantial sodium load. However, Hextend, a starch infusion delivered in a more physiological solution (ie, with a lower sodium content), is now available and a similar gelatin-based product is expected to become available within a year.
Complications of treatment
Numerous complications can occur as a result of fluid therapy. Perhaps the most obvious is the administration of too much fluid. When this occurs, the heart can fail to pump the expanded circulatory volume effectively. Over-distension of the left ventricle can cause heart failure and, consequently, pulmonary oedema. Patients suffering from this complication will display symptoms of a cough (producing a pink, frothy sputum) and respiratory distress — often worse when lying down. Renal failure and pre-existing ventricular impairment can exacerbate this condition.
Abdominal compartment syndrome and acute respiratory distress syndrome are both known consequences of excessive fluid resuscitation and fluid overload. Particular care has to be taken when treating any patient with co-existing cardiac or respiratory failure, or who is at risk of haemodynamic instability. By the time peripheral oedema or lung oedema are apparent, these patients have already been harmed by excessive volume or wrong choice of intravenous fluid.7,8
Biochemical disturbances
Biochemical abnormalities occur frequently in patients receiving IV fluid therapy and reflect the response to the fluid administered. Infusions of NaCl 0.9 per cent can result in over provision of sodium and chloride — the latter being a strong anion that can result in hyper-chloraemic acidosis (see Panel 3). In patients with underlying tendency to acidosis (eg, those with CO2 retention secondary to respiratory failure, or increased lactate levels following surgery), compensation mechanisms can easily be overwhelmed, resulting in severe metabolic acidosis.
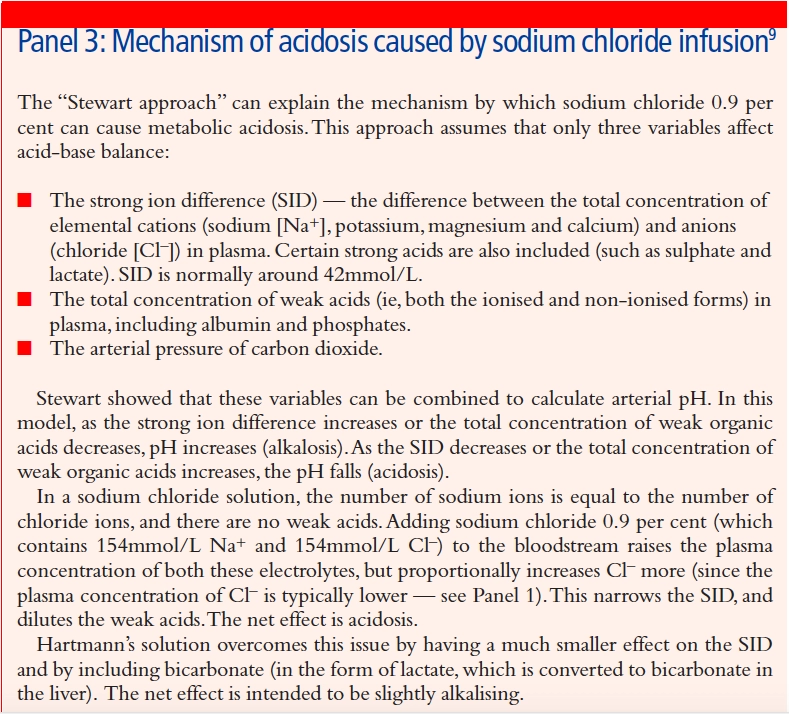
Risks are also associated with over-rapid correction of disturbed sodium levels. When using fluids to alleviate hypernatraemia, particularly of chronic duration (more than two days), the aim should be to reduce plasma sodium levels by no more than 0.5mmol/L per hour. This prevents the development of cerebral oedema.
Too rapid correction causes brain cells to shrink in response to the rapid rise of extra-cellular osmolality, resulting in a syndrome called central pontine myelinolysis. To avoid this, the absolute change in sodium levels should not exceed 20mmol/L during the first 48 hours of treatment.
Hypertonic saline solutions must not be administered in fluid-overloaded patients because they can precipitate heart failure. Hyponatraemia due to excess fluid shouldbe treated by fluid restriction or diuretics.
Haemodilution
Administering IV fluids in large volumes will inevitably lead to haemodilution. Following successful resuscitation, the resulting fall in haemoglobin levels usually corrects itself within a few days as the extra fluid is cleared by the kidneys. However, a blood transfusion may be required depending on the patient’s condition and local transfusion criteria.
Dilutional coagulopathy is another effect of high volume administration. In addition, some colloid infusions impair componentsof the clotting cascade. This is probably of less clinical consequence with colloids of smaller molecular size, but starches of higher molecular weight have been associated with increased bleeding. For example, dextran solutions are known antithrombotic agents and are mainly used nowadays for this indication.
Renal impairment
It has recently been suggested that starch solutions could potentially cause renal impairment. A possible explanation is hyperoncotic acute renal failure. If these products are given with insufficient water, the oncotic pressure of plasma is raised to the point where it effectively opposes the filtration pressure in the kidneys, thereby impairing normal glomerular filtration.
Current evidence for this suggests that certain types of HES are associated with increased morbidity. Although this may not be transferable to all starch infusions, serious consideration should be given before treating patients with large amounts of any HES.
Hypersensitivity
A further risk associated with colloids, particularly high molecular weight starches and dextrans, is the occurrence of hypersensitivities and anaphylactic reactions.
References
- Lobo DN, Dube MG, Neal KR, Simpson J, Rowlands BJ, Allison SP. Problems with solutions: drowning int he brine of an inadequate knowledge base. Clinical Nutrition 2001;20:125–30.
- Rivers E, Nguyen B, Hanstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. NewEngland Journal of Medicine 2001;345:1368–77.
- Dellinger RP, Levy MM, Carlet JM, Bion J, ParkerMM, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine 2008;36:296–327.
- Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews 2007, Issue 3.
- The SAFE study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. New England Journal of Medicine 2004;350:2247–56.
- Bunn F, Trivedi D, Ashraf S. Colloid solutions for fluid resuscitation. Cochrane Database of Systematic Reviews 2007, Issue 4.
- Grocott MPW, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesthesia and analgesia 2005;100:1093–106.
- Cotton BA, Guy JS, Morris JA, Abumrad NN. The cellular, metabolic and systemic consequences of aggressive fluid resuscitation strategies. Shock 2006;26:115–21.
- Morgan TJ. Clinical review: The meaning of acid-base abnormalities in the intensive care unit – effect of fluid administration. Critical Care 2005;9:204–11.

