CaptureItOne / Alamy Stock Photo
After reading this article, you should be able to:
- Explain the physiology of post-operative nausea and vomiting;
- Identify a patient’s risk, using the appropriate risk tool;
- List the management strategies recommended in the most recent guidelines.
Post-operative nausea and vomiting (PONV) is an umbrella term that covers nausea and vomiting occurring in the 24–48 hours following a surgical procedure[1,2]. Nausea and vomiting in combination are reflexes designed to protect against the absorption of toxins; however, olfactory, visual, vestibular and psychogenic triggers also exist, which have implications for hospital-ward environments.
Physiology
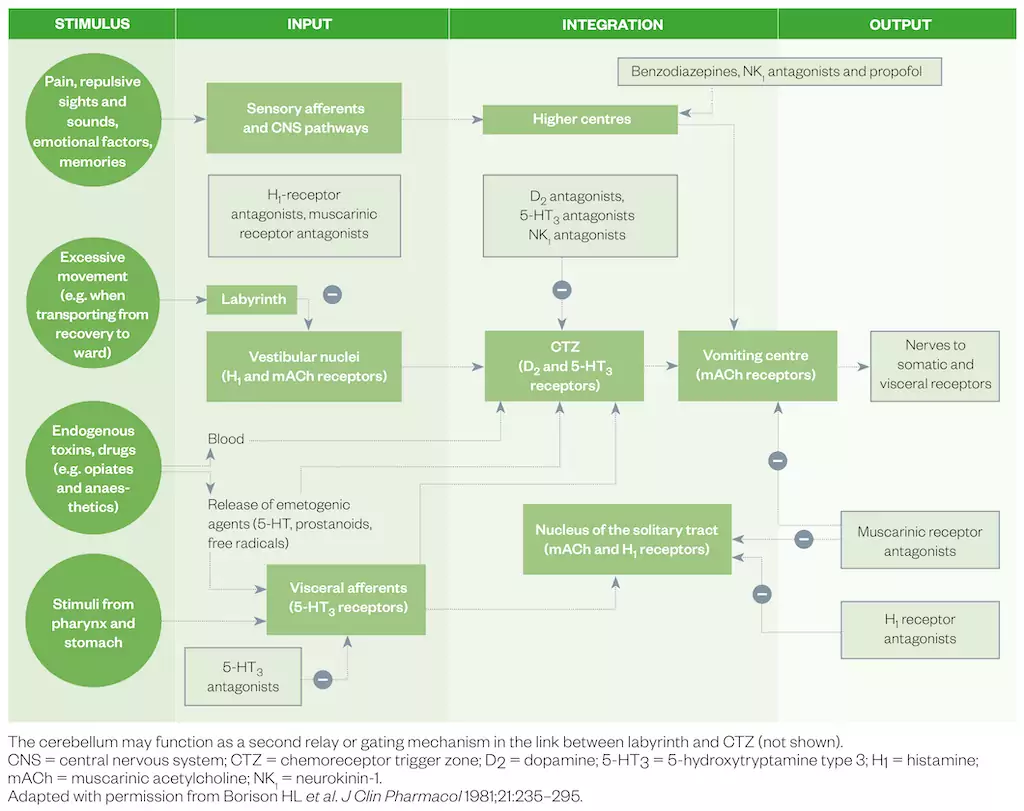
The pathophysiology of PONV is complex, involving both peripheral and central receptors. The ‘vomiting centre’ is activated by projections from the chemoreceptor trigger zone, cortical structures, gastrointestinal vagal afferents and the vestibular system[3–5]. In the peri-operative period, specific stimuli that activate these pathways include surgical trauma and inflammation, opioids, volatile anaesthetics and altered gastrointestinal motility[4]. The chemoreceptor trigger zone is outside the blood–brain barrier and can therefore be affected by opioids, volatile anaesthetics and neurotransmitters, such as 5-hydroxytryptamine (5-HT), which may be released during surgery. Figure 1 summarises the pathophysiology and site of action of clinically utilised medicines for PONV.
PONV is one of the most common adverse events in the post-operative period. Despite attempts to reduce its incidence, prevalence is estimated at 30% in the general surgical population and as high as 80% in high-risk cohorts[6]. It can be a highly distressing experience and is associated with significant patient dissatisfaction[7–10]. In addition, the occurrence of PONV is associated with a significantly longer stay in post-anaesthesia care units, unanticipated hospital admission and increased healthcare costs[11–13]. Given almost 3 million general anaesthetics are administered annually in the UK alone, the public health impact of reducing PONV is substantial[14].
In 2020, an international panel of renowned experts published the fourth version of their consensus guidelines for the management of PONV. The latest guidelines published by the Society for Ambulatory Anesthesia provide updated, evidence-based recommendations. These guidelines include risk stratification and prediction tools, interventions to reduce baseline risk, prophylaxis, optimal rescue treatment of established PONV and general implementation of PONV management in the context of the evidence base of pharmacological and non-pharmacological interventions[6].
Guideline 1: Identify patient’s risk of post-operative nausea and vomiting
The simplest and most widely used risk-scoring system for patients undergoing general anaesthesia is the Apfel risk score, which lists four risk factors[15]:
- Female gender;
- Non-smoking status;
- Use of post-operative opioids;
- Previous history of either PONV or motion sickness.
One point is given for the presence of each factor. The points can then be used to determine the approximate incidence (see Table 1[15]).
The Apfel risk score has been widely adopted because of its relative ease of application in everyday clinical practice; however, it does not take into account the much wider group of risk factors listed below[6]:
- Female sex;
- Type of surgery;
- Use of volatile anesthesia;
- History of post-operative nausea and vomiting;
- History of motion sickness;
- Non-smoking status;
- Duration of anaesthesia;
- Younger age;
- Post-operative opioid use.
Relying solely on risk scores is therefore likely to underestimate the occurrence of PONV. The updated guidance reflects this possibility and recommends a universal, multimodal approach to PONV prophylaxis for patients with at least one risk factor for PONV.
Guideline 2: Reduce baseline risk for post-operative nausea and vomiting
The baseline risk for PONV should be minimised by anaesthetic technique and avoiding well-known triggers where appropriate (see Box)[6].
Box: Strategies to reduce baseline risk of PONV used primarily during the intra-operative period
- Avoiding general anaesthesia by using regional anaesthesia instead;
- Using propofol for the induction and maintenance of anaesthesia;
- Avoiding nitrous oxide in surgeries lasting over one hour;
- Avoiding volatile anaesthetics;
- Minimising intra-operative and post-operative opioids (dose dependent);
- Ensuring adequate hydration (reduction of fasting period);
- Cost limiting until generic available at point of guideline review — using sugammadex instead of neostigmine for the reversal of neuromuscular blockade.
Strategies to reduce baseline risk for PONV are largely based in the intra-operative period, such as using propofol as the primary anaesthetic as part of total intravenous anaesthesia (TIVA) and avoiding volatile anaesthetics and nitrous oxide. Some strategies may continue from the intra-operative into the post-operative period, such as the use of multimodal analgesic regimens and opioid-sparing techniques, including use of paracetamol, alternative analgesic agents and preferential use of regional anaesthesia. Adequate hydration peri-operatively in patients undergoing same-day surgery is a simple yet effective strategy in reducing PONV[6].
Guideline 3: Administer post-operative nausea and vomiting (PONV) prophylaxis using two or more interventions in adults at risk of PONV
One of the major updates to the guidelines is the recommendation of multimodal anti-emetic prophylaxis, which in this context means administration of two anti-emetic agents from different medication classes to patients with at least one risk factor for PONV. If more than two risk factors are identified, three agents of differing medication classes must be considered on the basis of patient factors and medication safety profile. This change to the guidelines was prompted by concern over inadequate prophylaxis, as well as the limited availability of anti-emetic safety data.
Although dozens of different anti-emetics are available for clinical practice, there is currently no comparative ranking of the efficacy and safety of these medicines to inform clinical practice. A 2020 Cochrane systematic review with meta-analysis attempted to compare medications for preventing PONV by ranking them in terms of efficacy and safety — both single anti-emetic medicines and their combinations[16]. Combinations of medicines were generally more effective than the corresponding single medicines in preventing vomiting, with the exception of neurokinin-1 (NK1) receptor antagonists. This class of anti-emetics was found to be the most effective for prevention of vomiting, and these single medications demonstrated comparable efficacy to most of the anti-emetic combinations studied[16]. However, the study did note most of the clinical trials reviewed were conducted in patients at medium to high risk of nausea and vomiting; therefore, the benefit, or indeed risk, to patients outside of this group could not be determined.
Table 2 summarises evidence based anti-emetic dosing and optimum times for administration in adults[6]. Evidence of individual anti-emetics and combinations are discussed in detail within the consensus guidance and the reader is referred to this for further information. However, the principal message of the guidance is clear in that it does not matter which agent is selected for PONV prophylaxis, it is the number of agents administered that is of greater importance, provided each agent exerts its pharmacological effect via a different receptor site or mechanism of action. Clinicians’ choice of agent should also be considered in relation to the individual patient’s factors, along with the medication side effect profile. Side effects of prophylactic anti-emetics are rarely troublesome given that single administrations are required, often at low dose, but this should always be considered.
In clinical practice, many NHS trusts recommend using the corticosteroid dexamethasone and a 5-hydroxytryptamine (5-HT3) antagonist as first-line agents. Steroids are longer acting, negating the need for repeated administration. In addition, the use of 5-HT3 antagonists, usually ondansetron, are the gold standard, with extensive clinical evidence for use in PONV.
The mechanism of action of dexamethasone in PONV is not fully understood. It is thought to cause the inhibition of prostaglandin synthesis, by displaying anti-inflammatory efficacy and causing a decrease in the release of endogenous opiates[17]. Dexamethasone should be administered shortly after induction but, as its administration is associated with perianal pruritus, it should not be administered to patients while they are awake. The use of corticosteroids, primarily dexamethasone, intra-operatively for PONV is not associated with increased wound infection rates, anastomotic leak, wound healing, bleeding, or clinically significant hyperglycaemia[6].
Cyclizine is not recommended in the consensus guidelines, although dimenhydrinate and promethazine are mentioned. It must also be remembered that metoclopramide 10mg is largely ineffective, with a ‘number needed to treat’ of 30 for prophylaxis; therefore, it should not be included in local guidelines. Higher doses are more effective; however, metoclopramide tends to be avoided (unless a prokinetic effect is desired) in favour of the other dopaminergic agents[1,6,16].
NK1-receptor antagonist monotherapy seems to have similar efficacy to several combination therapies[16]. This class of medicines, however, is not licensed in the UK for prevention of PONV and has a higher cost. Instead, guidance suggests NK1-receptor antagonists may be useful prophylactic anti-emetics when post-operative emesis is highly undesirable, such as in gastric surgery and neurosurgery. Further study is also required on the effect of NK1-receptor antagonists on opioid requirements[6,16].
Non-pharmacological measures with an evidence base mentioned in the guidance include acupuncture and fluid management. Pericardium 6 acupuncture point stimulation is effective in preventing early PONV in adults with a ‘number needed to treat’ of approximately 4, although there is no benefit in children or in patients with late vomiting[18]. More practically, in clinical practice, adequate hydration is very effective in reducing the risk of PONV. This can be achieved by minimising peri-operative fasting time or using supplemental IV fluid to maintain clinical euvolemia. A 2019 Cochrane review demonstrated that supplemental crystalloids (10–30 mL/kg) reduce the risk of both early and late PONV, as well as the need for rescue anti-emetics[6,19].
Cost effectiveness
In addition to the evidence base, NHS trusts also need to consider both the cost and the availability of medicines. Medication shortages and high procurement costs for IV cyclizine and haloperidol have seen these options being removed from guidelines by trusts.
Figure 2 summarises the updated recommendations of the consensus guidelines for PONV management in adults[6].
Guideline 4: Administering prophylactic anti-emetic therapy to children at increased risk of post-operative nausea and vomiting
Paediatric PONV management is beyond the scope of this article; however, as noted in the consensus guidance, the principles are the same as in adult PONV management. Combination therapy is noted as being the most effective. Paediatric guidelines exclude children from birth to aged three years because of the rare incidence of PONV in children under the age of three years. Above this age, however, PONV rates are higher than in adults. This higher incidence reverts to adult rates after puberty.
Certain procedures, such as strabismus surgery, have a PONV rate of around 70% in children. These procedures are specifically included as risk factors in the stratification process. For high-risk children (those with three risk factors), TIVA is recommended with two anti-emetic agents — in contrast to the adult guidelines, where two risk factors or more warrant treatment with a minimum of three anti-emetics. Further information on medicines, dose ranges and the level of evidence can be found in the recent consensus guidelines[6].
Guideline 5: Provide anti-emetic treatment to patients with post-operative nausea and vomiting who did not receive prophylaxis or when prophylaxis failed
Very few prospective trials have studied rescue treatment of PONV after failure of prophylaxis, providing limited evidence to support clinical management. For many anti-emetics currently used in PONV rescue, significant uncertainty remains around the effective dose range, speed of onset, duration of effect, safety and overall risk–benefit ratio[20].
Figure 3 is based on a systematic review of the current clinical evidence and supports consensus guideline recommendations of using an agent acting on a different receptor to that used for prophylaxis[20]. Repeated administration should only be undertaken after medication levels have depleted or more than six hours have passed[7]. As with prophylaxis guidelines, use of combination therapy for treatment for PONV is recommended as being more effective and a 5-HT3 antagonist is recommended as the first-line choice in prophylaxis-naive patients.
Guideline 6: Ensure post-operative nausea and vomiting prevention and timely rescue treatment is implemented in the clinical setting
Adherence to PONV prophylaxis guidelines has been found to be remarkably low[6]. Less than half of medium- to high-risk patients receive the appropriate prophylaxis[6]. By making multimodal PONV prophylaxis an integral part of anaesthesia, the aim is to ensure better adherence through reducing the threshold for multimodal prophylaxis. PONV protocols and algorithms should ensure that an individual patient’s risk of PONV is assessed to identify the high-risk patients — those with more than one risk factor, who may require additional prophylaxis (i.e. three or more anti-emetic agents).
Post-operative nausea and vomiting guidelines
Any policy should include (as a minimum):
- Risk scoring;
- Other mechanisms to reduce baseline risk (see Box);
- Combination anti-emetic prophylaxis:
- Patient choice;
- Pre-existing conditions;
- Cost-effectiveness;
- Availability of drugs;
- Treatment of PONV.
Given that the evidence suggests compliance with PONV policy is poor, the Royal College of Anaesthetists suggests practice should be audited against local guidelines for PONV and provides relevant audit standards[21].
Guideline 7: Administer multimodal prophylactic anti-emetics in enhanced-recovery pathways
Enhanced-recovery pathways (ERP) are an evolving peri-operative concept in the UK. ERPs are an evidence-based approach that reduce variation in practice and aid patient recovery following major surgery. ERPs should include recommendations for PONV management as well as management of modifiable risk factors, discussed above.
In addition to the use of ERPs, pressure to perform more surgeries as day cases increases the risk of post-discharge nausea and vomiting, particularly if the patient has not been given adequate prophylaxis. This could result in poor patient satisfaction, readmission and increased use of healthcare resources and time.
Conclusion
PONV remains a significant clinical problem. In an attempt to reduce inadequate prophylaxis and poor compliance with local policy, the consensus guidance aims to implement multimodal prophylaxis in patients with at least one risk factor. Discretion based on patient and surgical risk factors, as well as local policy and medication availability, is advised.
Pharmacists working in the surgical environment should be aware that influencing PONV management is largely protocol-driven, requiring input and buy-in from all members of the multidisciplinary team caring for peri-operative patients. Attempts to manage change at ward level alone without input and buy-in are unlikely to succeed. Regular audit is necessary to monitor changes in practice.
This article was reviewed by Alanna Johnston and Sara Qureshi in May 2023, to ensure it remains relevant and up to date, following its original publication in The Pharmaceutical Journal in 2018.
- 1Wilson L, Knaggs R. The evidence base supporting pharmacological and non-pharmacological interventions for nausea and vomiting after surgery. Clin Pharm 2018;10:337–41.
- 2Pierre S, Whelan R. Nausea and vomiting after surgery. Continuing Education in Anaesthesia Critical Care & Pain. 2013;13:28–32. doi:10.1093/bjaceaccp/mks046
- 3Cao X, White PF, Ma H. An update on the management of postoperative nausea and vomiting. J Anesth. 2017;31:617–26. doi:10.1007/s00540-017-2363-x
- 4Horn CC, Wallisch WJ, Homanics GE, et al. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. European Journal of Pharmacology. 2014;722:55–66. doi:10.1016/j.ejphar.2013.10.037
- 5Wiesmann T, Kranke P, Eberhart L. Postoperative nausea and vomiting – a narrative review of pathophysiology, pharmacotherapy and clinical management strategies. Expert Opinion on Pharmacotherapy. 2015;16:1069–77. doi:10.1517/14656566.2015.1033398
- 6Gan TJ, Belani KG, Bergese S, et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesthesia & Analgesia. 2020;131:411–48. doi:10.1213/ane.0000000000004833
- 7Eberhart LHJ, Mauch M, Morin AM, et al. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57:1022–7. doi:10.1046/j.1365-2044.2002.02822.x
- 8Myles PS, Williams DL, Hendrata M, et al. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. British Journal of Anaesthesia. 2000;84:6–10. doi:10.1093/oxfordjournals.bja.a013383
- 9Macario A, Weinger M, Carney S, et al. Which Clinical Anesthesia Outcomes Are Important to Avoid? The Perspective of Patients. Anesthesia & Analgesia. 1999;89:652. doi:10.1097/00000539-199909000-00022
- 10Gan TJ, Sloan F, de L Dear G, et al. How Much Are Patients Willing to Pay to Avoid Postoperative Nausea and Vomiting? Anesthesia and Analgesia. 2001;:393–400. doi:10.1097/00000539-200102000-00022
- 11Habib AS, Chen Y-T, Taguchi A, et al. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Current Medical Research and Opinion. 2006;22:1093–9. doi:10.1185/030079906×104830
- 12Fortier J, Chung F, Su J. Unanticipated admission after ambulatory surgery — a prospective study. Can J Anaesth. 1998;45:612–9. doi:10.1007/bf03012088
- 13Hill RP, Lubarsky DA, Phillips-Bute B, et al. Cost-effectiveness of Prophylactic Antiemetic Therapy with Ondansetron, Droperidol, or Placebo. Anesthesiology. 2000;92:958–67. doi:10.1097/00000542-200004000-00012
- 14Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. British Journal of Anaesthesia. 2011;106:617–31. doi:10.1093/bja/aer058
- 15Apfel CC, Läärä E, Koivuranta M, et al. A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting . Anesthesiology. 1999;91:693–693. doi:10.1097/00000542-199909000-00022
- 16Weibel S, Rücker G, Eberhart LH, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database of Systematic Reviews. 2020;2020. doi:10.1002/14651858.cd012859.pub2
- 17Holte K, Kehlet H. Perioperative Single-Dose Glucocorticoid Administration: Pathophysiologic Effects and Clinical Implications. Journal of the American College of Surgeons. 2002;195:694–712. doi:10.1016/s1072-7515(02)01491-6
- 18Lee A, Chan SK, Fan LT. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2015;2016. doi:10.1002/14651858.cd003281.pub4
- 19Jewer JK, Wong MJ, Bird SJ, et al. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2019;2019. doi:10.1002/14651858.cd012212.pub2
- 20Gan TJ, Jin Z, Meyer TA. Rescue Treatment of Postoperative Nausea and Vomiting: A Systematic Review of Current Clinical Evidence. Anesthesia & Analgesia. 2022;135:986–1000. doi:10.1213/ane.0000000000006126
- 21Kumar A, Brampton W. Post-operative nausea and vomiting. In: Raising the Standard: a compendium of audit recipes for continuous quality improvement in anaesthesia . Royal College of Anaesthetists. 2012.https://www.rcoa.ac.uk/sites/default/files/documents/2019-09/CSQ-ARB-2012_0.pdf (accessed May 2023).