BSIP VEM / Science Photo Library
Overactive bladder (OAB) is defined as the combined presence of symptoms of urinary urgency, usually accompanied by frequency and nocturia (the need to wake in the night to urinate), with or without urgency incontinence, in the absence of urinary tract infection (UTI) or other obvious pathology[1]
. Based on work by FitzGerald and Brubaker, frequency is defined as increased daytime urinary frequency (with female patients reporting that urination occurs more often during waking hours than previously deemed normal) and is commonly viewed as more than seven voids per day[2]
.
This article aims to outline the pathophysiology and symptoms indicative of OAB, as well as the investigations required to confirm diagnosis and an overview of the evidence-based management options.
Epidemiology
Epidemiological studies in the United States report a prevalence of OAB in women of 16.9%, which increases to around 30.9% in women aged 65 years or over[3]
. Frequency was the most commonly reported symptom (85%), while 54% complained of urgency and 36% of urgency incontinence[3]
. European prevalence data indicate that the overall prevalence of OAB in people aged 40 years or over is 16.6%[4]
.
The European Prospective Investigation into Cancer and Nutrition (EPIC) study used a population-based, cross-sectional survey to estimate the prevalence of lower urinary tract symptoms (LUTS) in men and women aged over 18 years (n=19,165)[5]
. Around 11.8% of respondents complained of OAB symptoms and 64.3% reported at least one urinary symptom. Overall, OAB was found to be more prevalent than all types of urinary incontinence combined (9.4%), with nocturia the most commonly reported LUTS in 48.6% of men and 54.5% of women[5]
.
UK prevalence data from the EPIC study indicate that the overall prevalence of OAB is 8.7% in men and is 10.2% in women[5]
.
EPIC has been used to investigate the economic impact of OAB[6]
. Additional evidence demonstrates that OAB has a significant impact on work productivity, with people experiencing ‘wet’ OAB (i.e. urgency incontinence) reporting the lowest levels of work productivity and the highest levels of daily work interference[7]
.
Pathophysiology
Normal bladder function relies on normal structure (which is beyond the scope of this article) and a series of coordinated events to allow filling and voiding (see Figure 1). The vast majority (99%) of a normal micturation cycle is spent in the filling phase, which relies on the ability of the bladder to distend without increasing pressure (known as compliance). The nerve supply to the bladder is part of a reflex arc with afferent (sensory) and efferent (motor) fibres. Normal sensation allows the gradual awareness of filling. Interventions of higher centres through different spinal tracts suppress the feedback arm and reflex emptying (see Figure 2). Disruption of this process leads to symptoms of OAB; for example, central disruption leads to the loss of the reflex suppression and the start of reflex emptying (e.g. in patients with multiple sclerosis or stroke). Distal damage to the bladder through distension injuries, ageing or local insults (e.g. childbirth) lead to changes in both the afferent and efferent supply, and a replacement of the cholinergic fibres with nonadrenergic, noncholinergic fibres. These changes lead to a delay in the sensation of filling, increased end-organ sensitivity to acetylcholine (ACH) and also poorer detrusor contractility. The symptoms of OAB in people with underlying detrusor overactivity (UDO) are owing to involuntary contractions of the bladder muscle during the filling phase of the micturition cycle (see Figure 1)[1]
. These contractions are mediated by ACH-induced stimulation of bladder muscarinic receptors[8]
. However, OAB is not synonymous with detrusor overactivity as it is a symptom-based diagnosis, while UDO is a urodynamic observation. Around 64% of female patients with OAB have UDO and 83% of female patients with UDO have symptoms of OAB[9]
.
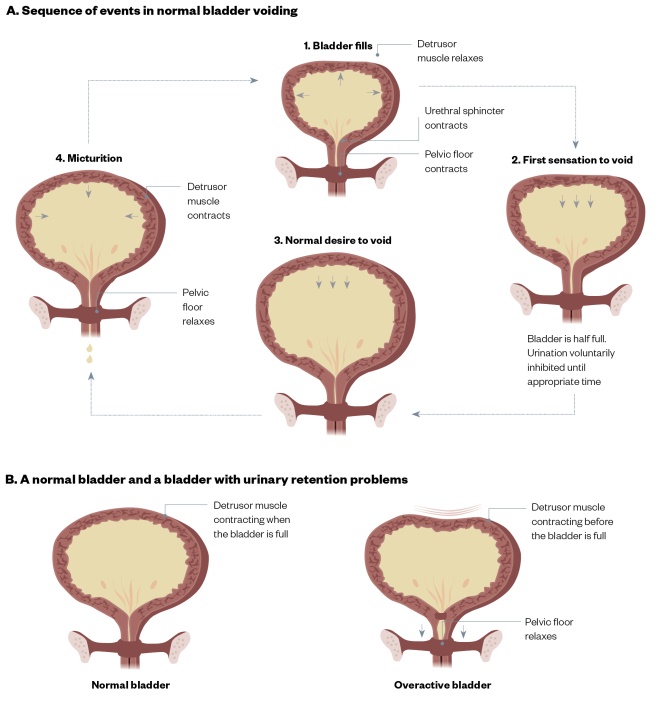
Figure 1: The micturation cycle
Source: JL / The Pharmaceutical Journal
A. Sequence of events in normal bladder voiding, and B. A normal bladder and a bladder with urinary retention problems
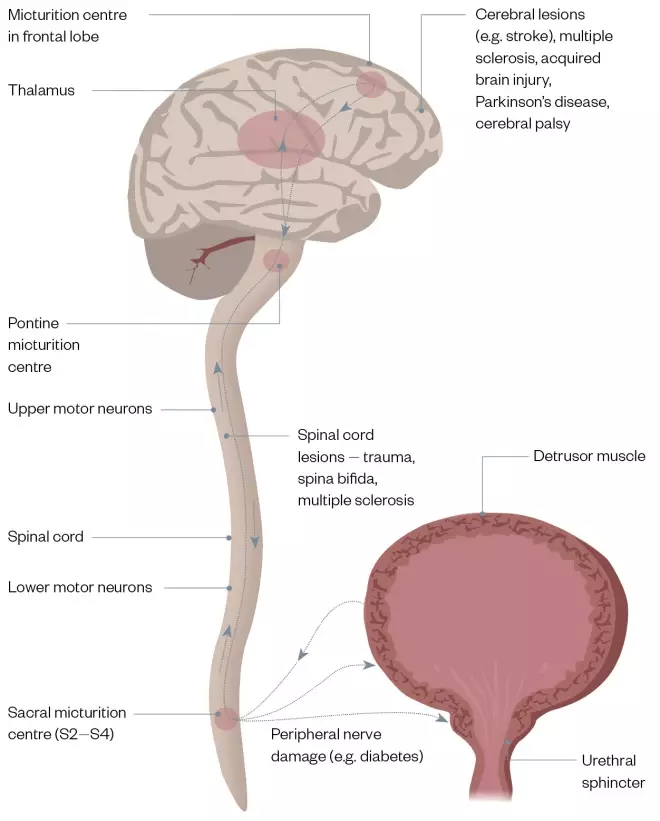
Figure 2: Nerve pathways that control micturition and the damage which may occur
Source: JL / The Pharmaceutical Journal
A digram showing the nerve pathways that control the micturation cycle and the potential damage that could impact on the cycle running normally
Muscarinic receptors
Molecular cloning studies have revealed that there are five distinct genes for muscarinic ACH receptors in humans and that there are five receptor subtypes (M1–M5)[10]
. In the human bladder, however, only mRNA-encoding M2 and M3 receptor subtypes have been identified[11]
. The M3 receptor is thought to be involved in direct smooth muscle contraction[12]
. The role of M2 receptors has not yet been elucidated, although it is thought that they may oppose sympathetically mediated smooth muscle relaxation or are used in the activation of nonspecific cationic channels and inactivation of potassium channels in the bladder[13]
. In general, M3 receptors are thought to be responsible for normal micturition contraction, although in certain disease states, such as neurogenic bladder dysfunction, M2 receptors may become more important in mediating detrusor contractions[14]
.
Clinical presentation
OAB is a term used to describe a collection of symptoms, rather than a specific diagnosis. Patients usually present with several symptoms, commonly urgency, daytime frequency, nocturia, urgency incontinence, stress incontinence, nocturnal enuresis (involuntary urination) and coital incontinence. However, it is important to remember that there are numerous other causes of urgency and frequency (see Box 1).
Box 1: Common causes of frequency and urgency of micturition
Urological
- Urinary tract infection;
- Detrusor overactivity;
- Small bladder capacity;
- Interstitial cystitis;
- Chronic urinary retention/chronic urinary residual;
- Bladder mucosal lesion (e.g. papilloma);
- Bladder calculus;
- Urethral syndrome;
- Urethral diverticulum;
- Urethral obstruction.
Gynaecological
- Pregnancy;
- Stress incontinence;
- Cystocoele;
- Pelvic mass (e.g. fibroids);
- Previous pelvic surgery;
- Radiation cystitis/fibrosis;
- Postmenopausal urogenital atrophy;
- Sexual arousal;
- Coitus;
- Sexually transmitted infection;
- Contraceptive diaphragm.
Medical
- Diuretic therapy;
- Upper motor neurone lesion;
- Impaired kidney function;
- Congestive cardiac failure (nocturia);
- Hypokalaemia;
- Endocrine (there are several hormones that have an effect on urgency, but they are beyond the scope of this article);
- Diabetes mellitus;
- Diabetes insipidus;
- Hypothyroidism;
- Psychological (there are several psychological causes that have an effect on urgency, but they are beyond the scope of this article);
- Excessive drinking;
- Habit;
- Anxiety.
Vulval excoriation (skin picking), urogenital atrophy, and urinary residual and stress incontinence can be indicative of OAB in women; however, there are no specific clinical signs.
Investigation
All patients require a basic assessment in order to exclude any other underlying cause for LUTS and a midstream urine sample should be sent for microscopy, culture and sensitivity to exclude a lower UTI.
Patients should also be advised to complete a frequency/volume chart or bladder diary in order to evaluate their fluid intake and voiding pattern. As well as the number of voids and incontinence episodes, the mean volume voided over a 24-hour period can be calculated, in addition to the diurnal and nocturnal volumes. Urgency is now generally regarded as being the driving symptom of OAB and is known to play an important role in the development of daytime frequency, nocturia and urgency incontinence. Several validated urgency scoring systems, including the Patient Perception of Intensity of Urgency Scale[15]
, Urgency Perception Score[16]
and Indevus Urgency Severity Scale[17]
have been developed to attempt to measure urgency severity, and these may be used alongside frequency volume charts in clinical practice.
The patient’s quality of life is assessed using questionnaires, completed alone or as part of consultation with their GP or specialist. These questionnaires allow quantification of morbidity and treatment efficacy to be evaluated, while measuring the effect on daily living and the coping strategies adopted.
Pharmacy professionals may be aware of generic questionnaires, such as Short Form 36[18]
, which use general measures of health status to determine quality of life and are therefore applicable to a wide range of populations and clinical conditions. Disease-specific questionnaires, such as the King’s Health Questionnaire[19]
, have been designed to focus on LUTS. More recently, the International Consultation on Incontinence has developed and validated several disease-specific questionnaires for use in lower urinary tract dysfunction[20]
.
Urodynamic investigations
While some female patients with symptoms suggestive of OAB can be managed following basic assessment, whether in pharmacy or in combination with general practice, women with refractory or complex symptoms may benefit from urodynamic investigations. Basic urodynamic investigations include uroflowmetry, filling cystometry and pressure/flow voiding studies. However, when these tests do not replicate, the patient’s symptoms, video urodynamics or ambulatory urodynamics may be more informative. These are specialist investigations employed in specialist units[21]
.
Cystourethroscopy
Cystourethroscopy is an endoscopic procedure using either a rigid or flexible tube (cystoscope) that is inserted through the urethra and into the bladder. Sterile water runs through the cystoscope to allow for a more comfortable and more complete visual examination.
Although endoscopy is not helpful in diagnosing OAB, it may be used to exclude other causes for the symptoms associated with OAB, such as a bladder tumour or calculus. In addition, cystourethroscopy should be considered in all women complaining of haematuria, painful bladder syndrome, recurrent incontinence or recurrent UTIs.
Lifestyle factors and conservative management
All women with OAB benefit from advice on simple measures that can help alleviate their symptoms. Many patients drink too much fluid and should be advised to reduce their fluid intake to between 1L and 1.5L per day[22]
, and to avoid tea, coffee and alcohol if these exacerbate their symptoms. Smoking can also be a contributory factor, so patients should be advised and supported to reduce or quit smoking. Weight loss in patients who are obese may also help improve symptoms of urinary incontinence[23]
. Simple tips to encourage weight loss include eating a balanced healthy diet and swapping sugary fizzy drinks for alternatives with fewer or no calories, such as ‘diet’ versions or sparkling water with a slice of lemon. A combination of following a healthy diet with an appropriate exercise routine may help people to maintain weight loss, so it may also be appropriate to signpost patients to local swimming pools or parks within the community, or weight-loss groups or initiatives that could be helpful in promoting a healthier lifestyle.
Bladder retraining
Bladder retraining is the term used to describe the process of re-educating the bladder by timed voids, first described by Jeffcoate & Francis[24]
. It can be effective, if delivered in the inpatient and outpatient setting. The meta-analysis by Berghmans et al. concluded that bladder retraining is more effective than placebo and drug therapy
[25]
. It also demonstrated that there is insufficient evidence to support the effectiveness of electrical stimulation, and too few studies to evaluate the effect of pelvic floor exercises and biofeedback (where computer graphs and audible tones are used to demonstrate the muscles used during exercise) in women with urgency urinary incontinence[25]
. The National Institute for Health and Care Excellence[26]
and the International Consultation on Incontinence[27]
recommend that bladder retraining should be considered as first-line treatment in all women with OAB.
When added to nondrug therapy, antimuscarinic therapy can be useful for the management of patients with OAB, as it acts to reduce involuntary detrusor contractions and increase bladder capacity. A Cochrane review of 23 trials (n=3,685 patients) showed that symptomatic improvement was more common among those receiving antimuscarinic therapy compared with bladder retraining (relative risk [RR] 0.74; 95% confidence interval [CI] 0.61–0.91) and that combination treatment was also associated with more improvement than bladder training alone (RR 0.57; CI 0.38–0.88). Similarly, there was a trend towards greater improvement in symptoms with the use of combination antimuscarinic therapy and bladder retraining compared with antimuscarinic therapy alone (RR 0.80; CI 0.62–1.04), although this was not statistically significant[28]
.
Medical management
While a conservative approach is justified initially, drug therapy remains integral in the management of women with OAB and there are currently several different antimuscarinic drugs available, as well as the newer beta-3-agonist, mirabegron.
Antimuscarinics
Traditionally, tolerability, compliance and persistence have limited the usefulness of many antimuscarinic agents; however, with the introduction of newer bladder-selective drugs, once-daily dosing and differing routes of administration, it is possible that persistence with therapy may increase in the future.
Generally, the ‘beneficial’ antimuscarinic effects are initiated through the M3 receptor, with many of the unwanted side effects initiated through the M1 receptor. Darifenacin also exerts effects on the M2 receptor, which is intimate with the beta-3-receptor (which encourages bladder relaxation rather than blocking the M3 receptor involved in active bladder contraction). Tolterodine is also regarded as bladder-selective rather than receptor-specific and trospium, as a larger quaternary amine, is less lipophilic and does not cross the blood–brain barrier[29]
.
There are now several licensed antimuscarinic drugs available on the market. These have all been reviewed by the International Consultation on Incontinence[30]
(see Table) and have Level 1 (the highest level) evidence[31]
and a Grade A recommendation[32]
. Box 2 provides a list of common side effects that can be managed by altering the medication (e.g. constipation can be reduced by using the transdermal route for oxybutynin[33]
, cognition may be less at risk using fesoterodine[34]
and solifenacin may be more M3 specific[35]
).
| Antimuscarinic drugs | Level of evidence[31] | Grade of recommendation[32] |
|---|---|---|
| Darifenacin | 1 | A |
| Fesoterodine | 1 | A |
| Oxybutynin | 1 | A |
| Propiverine | 1 | A |
| Solifenacin | 1 | A |
| Tolterodine | 1 | A |
| Trospium | 1 | A |
Box 2: Common side effects of anticholinergic medicines
A systematic review and meta-analysis of 83 studies (n=30,699 participants) of six drugs (fesoterodine, oxybutynin, propiverine, solifenacin, tolterodine and trospium) supports the efficacy of antimuscarinic therapy for OAB. Overall, there was a significantly higher return to continence, favouring active treatment over placebo; the pooled RR across different studies and drugs was 1.3–3.5 (P <0.01). Antimuscarinic therapy was also more effective in reducing the number of incontinence episodes, micturitions and urgency episodes per day — a finding that was also statistically significant[38]
.
Anticholinergic burden
While antimuscarinic therapy remains integral in the management of women with OAB, increasing evidence suggests that these drugs may act on the central nervous system, leading to a long-term reduction of cognitive function and the development of dementia[39]
.
A systematic review of 46 studies (n=60,944 participants) demonstrated a significant decline in cognitive ability with increasing anticholinergic load and an increasing trend in mortality, although this was not found to be statistically significant[40]
. These findings were supported by a two-year longitudinal study of 13,004 participants aged over 65 years who were taking anticholinergic medication. Overall, use of drugs with an anticholinergic effect was associated with a 0.33 point decline in the Mini Mental State Examination (CI 0.03–0.64; P =0.03) and an increased risk in terms of two-year mortality (odds ratio 1.68; CI 1.30–2.16; P <0.001)[41]
. This suggests that anticholinergic drugs should be used with caution in older people.
Further evidence is provided by a large prospective cohort study from North America investigating the association of total standardised daily dose of anticholinergic and the onset of dementia and Alzheimer’s disease[42]
. Overall, a ten-year dose–response relationship was observed for both dementia and Alzheimer’s disease (test for trend P <0.001), with the greatest risk being associated with the highest anticholinergic dose (adjusted hazard ratio 1.54; 95% CI 1.21–1.96)[42]
.
While the use of antimuscarinic medicine is not contraindicated in older people, it is important to be aware of comorbidities, particularly the risk of polypharmacy, before treating OAB. Many medicines exert anticholinergic effects and it is important to be aware of this prior to initiating therapy in order to reduce the overall anticholinergic load; this may be assessed clinically using an anticholinergic burden scale[37]
.
Beta adrenoceptors
Adrenoceptors are members of a family of seven transmembrane receptors. The two main groups, alpha and beta, each have several subtypes. Beta-1-, beta-2- and beta-3-adrenoceptors have been identified in human urothelium and detrusor muscle, with beta-3 being highly expressed in the bladder[43],[44]
.
Beta-3-adrenoceptors are the predominant subtype, with 95% of all beta-adrenoceptor mRNA in the human bladder relating to the beta-3 subtype; this receptor is thought to be important for mediating human detrusor relaxation[45],[46]
. This is supported by both animal and in vitro studies[47],[48]
.
Mirabegron
Mirabegron is the first commercially available selective beta-3-agonist for the treatment of OAB. In a randomised, double-blind, placebo-controlled North American trial of 1,329 patients with OAB[49]
, mirabegron 50mg and 100mg demonstrated significantly greater efficacy than placebo for the coprimary endpoints of incontinence episodes and micturition frequency. Since mirabegron is an adrenoceptor agonist, it does not have the typical adverse effects associated with antimuscarinic agents (e.g. dry mouth rates were 1.5%, 0.5% and 2.1% in the placebo, 50mg and 100mg groups, respectively). No differences were found in the incidence of hypertension between the mirabegron arm and the placebo arm[49]
.
The long-term safety of mirabegron was examined in a 12-month randomised, double-blind study in 2,444 patients using mirabegron 50mg, 100mg and tolterodine extended-release 4mg as an active comparator[50]
. Dry mouth was reported in 2.8%, 2.3% and 8.6% of patients, respectively, while mean changes in systolic blood pressure were 0.2mmHg, 0.4mmHg and –0.5mmHg, respectively[50]
.
The licensed dose of mirabegron is 50mg daily, although this should be reduced to 25mg daily in patients with renal or hepatic impairment. Mirabegron remains an option where anticholinergics are contraindicated, have failed or have had limited benefit.
Combination therapy: mirabegron and solifenacin
The efficacy and safety of combination therapy with solifenacin and mirabegron in patients with an inadequate response to solifenacin monotherapy has been investigated (BESIDE study)[51]
. BESIDE was a prospective study of 2,174 patients refractory to solifenacin 5mg daily monotherapy who were randomised to combination therapy (solifenacin 5mg and mirabegron 50mg) or solifenacin monotherapy (5mg or 10mg). The study results indicated that the efficacy of combination therapy was superior with significant improvements in incontinence episodes (P =0.001) and micturition frequency (P <0.001). Combination therapy was non-inferior to solifenacin 10mg for micturition frequency and incontinence episodes over three days[51]
.
While the results of the BESIDE study clearly demonstrate the efficacy of combination therapy in women with refractory symptoms, the use of primary combination therapy is less clear. The SYNERGY study was a prospective, double-blind randomised-controlled trial (RCT) investigating the use of combination therapy (solifenacin 5mg and mirabegron 25mg or 50mg) compared with monotherapy in 3,398 patients in 435 sites in 42 countries[52]
. Overall, combination therapy with solifenacin 5mg and mirabegron 25mg, and solifenacin 5mg and mirabegron 50mg provided consistent improvements in efficacy compared with monotherapy, with patient-reported outcome measures and health-related quality of life also showing a clear benefit of combination, demonstrating a meaningful effect to patients[53]
.
Oestrogen
The effect of oestrogens on lower urinary tract function remain controversial; however, evidence from animal studies has suggested that oestrogen deficiency may increase the risk of developing OAB following the menopause[54]
. In vitro studies using tissues from ovariectomised rats have shown a significant decrease in voided volume and an increase in urinary frequency, with increased basal and stretch-induced acetylcholine release. Conversely, there was a reduction in acetylcholine release from nerve fibres[54]
. This may explain why there is a decrease in detrusor contractility following the menopause with a corresponding increase in the development of OAB symptoms. Oestrogen replacement therapy reversed these changes[55]
.
Given the concerns regarding the use of systemic oestrogen replacement therapy, the vaginal route of administration may offer a better treatment approach.
Oestrogens have been used in the treatment of urinary urgency and urgency incontinence for many years, although there have been few controlled trials to confirm their efficacy. In a review of ten randomised, placebo-controlled trials, oestrogen was found to be superior to placebo when considering symptoms of urgency incontinence, frequency and nocturia, although vaginal oestrogen administration was found to be superior to placebo for the symptom of urgency[56]
.
Combination therapy: oestrogens and antimuscarinics
There is emerging evidence regarding the synergistic use of vaginal oestrogen therapy with antimuscarinic medicine in the management of postmenopausal women with OAB, although the results are contradictory.
One RCT compared tolterodine and vaginal conjugated oestrogen cream versus tolterodine alone[57]
. It showed those women receiving combination therapy had a significantly greater improvement in mean daytime frequency and voided volume compared with the tolterodine arm. These observations were supported by a significantly greater improvement in health-related quality of life in the combination therapy group[57]
.
A further RCT compared the oestradiol-releasing vaginal ring (Estring®, Pfizer) and oxybutynin[58]
. The oxybutynin group had a mean decrease of 3.0 voids per day compared with a decrease of 4.5 voids in those using the oestradiol ring, although the difference was not statistically significant. In addition, there was a significant improvement in quality of life in both groups, although again with no difference between the groups[58]
.
These findings have been supported by two further studies demonstrating the synergistic effect of treatment with solifenacin[59]
and fesoterodine[60]
with vaginal oestrogens in patients with OAB.
However, these findings in patients with OAB have not been replicated in a prospective study of postmenopausal women with an UDO treated with tolterodine extended release, with or without vaginal oestriol[61]
. Overall, there were no significant differences between the two treatment groups in terms of efficacy.
Desmopressin
1-desamino-8-D-arginine vasopressin (demopressin) is a synthetic vasopressin analogue that has strong antidiuretic effects without altering blood pressure. It has been used primarily in the treatment of nocturia and nocturnal enuresis in children[62]
and adults[63]
. More recently, nasal desmopressin has been reported as a ‘designer drug’ for the treatment of daytime urinary incontinence[64]
. It is safe for long-term use; however, the drug should be used with care in older people owing to the risk of hyponatraemia.
In addition, in those patients with symptomatic nocturia secondary to underlying nocturnal polyuria (defined as more than a third of a person’s urine being produced at night), it may have a role in reducing nocturia[65]
. A new ultra-low-dose formulation has recently been introduced that potentially reduces the risk of hyponatraemia[66]
.
Referring to secondary care: refractory OAB
While the majority of patients with OAB will respond to conservative therapy and drug treatment, a minority will continue to complain of distressing lower urinary tract symptoms. These patients benefit from referral to secondary care for further management where more invasive therapy (e.g. intravesical botulinum toxin or neuromodulation) may be indicated.
Botulinum toxin
Intravesical botulinum toxin administered by injection at cystoscopy offers an alternative in patients with intractable OAB, with the effect lasting 9–12 months. Onabotulinum toxin works by binding to the presynaptic ‘SNARE’ proteins, which are critical for the presynaptic vesical release of acetylcholine into the synaptic cleft[67]
. The neurotoxin forms a permanent bond trapping acetylcholine in the vesicle. There is a risk of voiding difficulties, which appears to be related to dose. Current evidence suggests that repeat procedures are safe and remain effective[68]
. Recovery from onabotulinum toxin requires the sprouting of new synapses. This process classically takes around 8–12 weeks, which is why the cosmetic effects of muscle paralysis wear off typically in this time frame and the procedure needs to be repeated. However, onabotulinum toxin also has effects on secondary messengers that are involved in neuromodulation. These effects are not well understood, but the effects are longer lasting and explain why pain (in particular) improves immediately rather than being gradual, as seen with the 48–72 hours required for flaccid paralysis. The effect of onabotulinum toxin on bladder function usually lasts for 9–12 months.
Neuromodulation
Neuromodulation may also be used in women with refractory symptoms and may be peripheral or central.
Peripheral neuromodulation: percutaneous tibial nerve stimulation
The tibial nerve is a mixed nerve containing L4-S3 fibres and originates from the same spinal cord segments as the innervation to the bladder and pelvic floor. Peripheral neural modulation may, therefore, have a role in the management of urinary symptoms.
Percutaneous tibial nerve stimulation (PTNS) is achieved by the temporary insertion of a needle in the lower leg, posterior to the tibia, above the medial malleolus. Treatment is performed in the clinic setting and begins with weekly treatment for the first 12 weeks, after which, maintenance therapy is generally monthly, with each session lasting 30 minutes.
PTNS has been shown to be a safe and effective treatment, and comparable to that of pharmacotherapy[69]
. A recent systematic review and meta-analysis[70]
reported a pooled subjective success rate of 61.4% (CI 57.5–71.8) and an objective success rate of 60.6% (CI 49.2–74.7)[70]
. A significant drawback of PTNS in treating a chronic condition, such as OAB, is the need for repeated stimulations, as symptoms deteriorate by 6–12 weeks[71]
. There are limited long-term data in the literature, with few studies looking at ongoing treatment over 12 months. A recent study has shown PTNS therapy as a safe, durable and valuable long-term treatment option for OAB symptom control[72]
.
Sacral neuromodulation
Sacral nerve stimulation (SNS) has emerged as an important potential therapeutic option for refractory OAB. More than 50,000 patients have received SNS worldwide for the treatment of several lower urinary tract symptoms since it was introduced in 1997. SNS uses a surgically implanted lead and generator to stimulate the S3 sacral nerve root. The stimulation of afferent nerve fibres modulates reflex pathways involved in the filling and evacuation phase of micturition through spinal circuits mediating somatovisceral interactions within the sacral spinal cord. SNS is thought to activate or ‘reset’ the somatic afferent inputs that play a central role in the modulation of sensory processing and micturition reflex pathways in the spinal cord[73]
.
This treatment option incorporates a temporary test stimulation that allows patients and physicians to assess SNS over a trial period[74]
. If the patient’s symptoms improve by at least 50% then the patient is a candidate to undergo the second stage, or permanent step, in which the permanent implantable pulse generator is implanted in the soft tissue of the patient’s buttock.
SNS has been shown to be effective in more than 40 studies. Most of these studies define success as greater than 50% improvement in clinical symptoms. While the reported success rates for subjects who actually received the implantation varied between 60% and 100%, an intention-to-treat analysis in a recent systematic review revealed success rates of 21–48% for stage 1 implantation with percutaneous nerve evaluation and of 75–80% for stage 2 implantation[75]
.
Long-term follow-up reported gradual decrease of the success rate from 87% at 1 month to 62% at 5 years[76]
.
Reconstructive surgery
Traditional operations, including clam cystoplasty (a major operation to increase the size of the bladder), substitution cystoplasty and ileal conduit, continue to have a small role for truly refractory cases, although their use has decreased to a handful of cases owing to the advent of nerve stimulation and onabotulinum toxin.
Conclusion
OAB is a common and distressing condition that is known to have a significant effect on health-related quality of life. The clinical diagnosis of OAB is often one of exclusion. The majority of female patients will benefit from conservative measures in the first instance, although many will eventually require drug therapy. For those with refractory symptoms, switching to an alternative class of therapy may be useful and there is now considerable evidence to support the use of combination therapy in those women with persistent symptoms. If pharmacotherapy fails, more invasive therapy such as intravesical botulinum toxin[77]
, PTNS[78]
or sacral neuromodulation[79]
can be used.
Systemic oestrogens do not have a role in treating OAB; however, vaginal oestrogens may be helpful and may also act synergistically with antimuscarinic drugs.
Financial and conflicts of interest disclosure
Philip Toozs-Hobson has acted as an adviser to Contura and speaker for Pierre Fabre and Ferring. Dudley Robinson has been a speaker for Ferring, Astellas, Ixaltis and Allergan. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in, or financial conflict with the subjet matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
References
[1] Haylen BT, de Ridder D, Freeman RM et al. An International Urogynaecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 2010;21:5–26. doi: 10.1007/s00192-009-0976-9
[2] FitzGerald MP & Brubaker L. Variability of 24-hour voiding diary variables amongst asymptomatic women. J Urol 2003;169(1):207–209. doi: 10.1016/S0022-5347(05)64069-4
[3] Stewart WF, Corey R, Herzog AR et al. Prevalence of overactive bladder in women: results from the NOBLE program. Int Urogynaecol J 2001;12(3):S66
[4] Milsom I, Abrams P, Cardozo L et al. How widespread are the symptoms of overactive bladder and how are they managed? A population-based prevalence study. BJU Int 2001;87(9):760–766. PMID: 11412210
[5] Irwin DE, Milsom I, Hunskaar S et al. Population–based survey of urinary incontinence, overactive bladder and other lower urinary tract symptoms in five countries; results of the EPIC study. Eur Urol 2006;50:1306–1314. doi: 10.1016/j.eururo.2006.09.019
[6] Irwin DE, Mungapen L, Milsom I et al. The economic impact of overactive bladder syndrome in six Western countries. BJU Int 2009;103:202–209. doi: 10.1111/j.1464-410X.2008.08036.x
[7] Sexton CC, Coyne KS, Vats V et al. Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care 2009;15:S98–S107. PMID: 19355804
[8] Anderson KE. The overactive bladder: pharmacologic basis of drug treatment. Urology 1997;50(6A Suppl):74–84. PMID: 9426756
[9] Hashim H & Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? J Urol 2006;175(1):191–195. doi: 10.1016/S0022-5347(05)00067-4
[10] Caulfield MP & Birdsall NJ. International Union of Pharmacology XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998;50(2):279–290. PMID: 9647869
[11] Yamaguchi O, Shisida K, Tamura K et al. Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. J Urol 1996;156(3):1208–1213. PMID: 8709348
[12] Harris DR, Marsh KA, Birmingham AT et al. Expression of muscarinic M3 receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. J Urol 1995;154(3):1241–1245. PMID: 7637095
[13] Hedge SS, Choppin A, Bonhaus D et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol 1997;120(8):1409–1418. doi: 10.1038/sj.bjp.0701048
[14] Braverman AS & Ruggieri MR. M2 receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol 1998;275(5 Pt 2):R1654–1660. PMID: PMC3275805
[15] Cartwright R, Panayi D, Cardozo L & Khullar V. Reliability and normal ranges for the patient’s perception of intensity of urgency scale in asymptomatic women. BJU Int 2010;105(6):832–836. doi: 10.1111/j.1464-410X.2009.08846.x
[16] Cardozo L, Coyne KS & Versi E. Validation of the urgency perception scale. BJU Int 2005;95(4):591–596. doi: 10.1111/j.1464-410X.2005.05345.x
[17] Nixon A, Colman S, Sabounjian L et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol 2005;174(2):604–607. doi: 10.1097/01.ju.0000165461.38088.7b
[18] Jenkinson C, Coulter A & Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993;306(6890):1437–1440. PMID: PMC1677870
[19] Kelleher CJ, Cardozo LD, Khullar V & Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 104(12):1374–1379. PMID: 9422015
[20] Castro Diaz D, Robinson D, Bosch R et al. 5B Patient reported outcome assessment. In: Abrams P, Cardozo L, Wagg A & Wein A, ed. Incontinence, 6th Edition. Bristol: International Continence Society; 2017. 541–599
[21] Chester J, Toozs-Hobson P & Israfil-Bayli F. The role of ambulatory urodynamics in investigation of female urinary incontinence. Int Urogynecol J 2016;27(3):381–386. doi: 10.1007/s00192-015-2817-3
[22] Swithinbank L, Hashim H & Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol 2005;174(1):187–189. doi: 10.1097/01.ju.0000162020.10447.31
[23] Subak LL, Wing R, West DS et al.; PRIDE investigators. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med 2009;360(5):481–490. doi: 10.1056/NEJMoa0806375
[24] Jeffcoate TN & Francis WJ. Urgency incontinence in the female. Am J Obstet Gynecol 1966;94(5):604–618. PMID: 5906586
[25] Berghmans LC, Hendricks HJ, de Bie RA et al. Conservative treatment of urge urinary incontinence in women: a systematic review of randomised clinical trials. BJU Int 2000;85(3):254–263. PMID: 10671878
[26] National Institute for Health and Care Excellence. The management of urinary incontinence in women. Clinical guideline [CG171] 2015. Available at: https://www.nice.org.uk/guidance/cg171 (accessed March 2019)
[27] Dumoulin C, Adewuyi T, Booth J et al. Adult Conservative Management. In: Abrams P, Cardozo L, Wagg A & Wein A, ed. Incontinence, 6th Edition. Bristol: International Continence Society; 2017. 1443–1629.
[28] Prasad Rai B, Cody JD, Alhasso AA & Stewart L. Anticholinergic drugs versus non drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst Rev 2012;12:CD003193. doi: 10.1002/14651858.CD003193.pub4
[29] Cardozo L, Chapple CR, Toozs-Hobson P et al. Efficacy of trospium chloride in patients with detrusor instability: a placebo-controlled, randomized, double blind multicentre clinical trial. BJU 2000;85:659–664. PMID: 10759661
[30] Andersson KE, Cardozo L, Cruz F et al. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Wagg A & Wein A, ed. Incontinence, 6th Edition. Bristol: International Continence Society; 2017. 805–958
[31] Hadorn DC, Baker D, Hodges JS & Hicks N. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 1996;49(7):749–754. PMID: 8691224
[32] Harbour R & Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001;323(7308):334–336. PMID: PMC1120936
[33] Cohn JA, Brown ET, Reynolds WS et al. An update on the use of transdermal oxybutynin in the management of overactive bladder disorder. Ther Adv Urol 2016;8(2):83–90. doi: 10.1177/1756287215626312
[34] Heesakkers J, Espuña Pons M, Toozs Hobson P & Chartier-Kastler E. Dealing with complex overactive bladder syndrome patient profiles with focus on fesoterodine: in or out of the EAU guidelines? Res Rep Urol 2017;9:209–218. doi: 10.2147/RRU.S146746
[35] Chapple CR, Martinez-Garcia R, Selvaggi L et al. A comparision of the efficacy & tolerability of solifenacin succinate & extended release tolterodine at treatment of overactive bladder results of the STAR trial. Eur Urol 2005;48(7):464–470. doi: 10.1016/j.eururo.2005.05.015
[36] Bulchandani S, Toozs-Hobson P, Parsons M et al. Effect of anticholinergics on the overactive bladder and bowel domain of the electronic personal assessment questionnaire (ePAQ). Int Urogynecol J 2015;26(4):533–537. doi: 10.1007/s00192-014-2527-2
[37] Pharmaceutical Services Negotiating Committee. Anticholinergic burden scale. 2012. Available at: https://psnc.org.uk/lancashire-lpc/wp-content/uploads/sites/97/2014/02/Anticholinergic-burden-scale-2012.pdf (accessed March 2019)
[38] Chapple CR, Khullar V, Gabriel Z et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 2008;54(3):543–562. doi: 10.1016/j.eururo.2008.06.047
[39] Araklitis G, Thiagamoorthy G, Hunter J et al. Anticholinergic prescription: are healthcare professionals the real burden? Int Urogynaecol J 2017;28(8):1249–1256. doi: 10.1007/s00192-016-3258-3
[40] Fox C, Smith T, Maidment I et al. Effect of medications with anticholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing 2014;43(5):604–615. doi: 10.1093/ageing/afu096
[41] Fox C, Richardson K, Maidment ID et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc 2011;59:1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x
[42] Gray SL, Anderson ML, Dublin S et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015;175:401–407. doi: 10.1001/jamainternmed.2014.7663
[43] Andersson KE & Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 2004;84(3):935–986. doi: 10.1152/physrev.00038.2003
[44] Otsuka A, Shinbo H, Matsumoto R et al. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol 2008;377(4–6):473–481. doi: 10.1007/s00210-008-0274-y
[45] Takeda M, Obara K, Mizusawa T et al. Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 1999;288(3):1367–1373. PMID: 10027879
[46] Nomiya M & Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol 2003;170(2 Pt 1):649–653. doi: 10.1097/01.ju.0000067621.62736.7c
[47] Sacco E, Bientinesi R, Tienforti D et al. Discovery history and clinical development of mirabegron for the treatment of overactive bladder and urinary incontinence. Expert Opin Drug Discov 2014;9(4):433–448. doi: 10.1517/17460441.2014.892923
[48] Hicks A, McCafferty GP, Riedel E et al. GW427353 (solabegron), a novel, selective beta3-adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog. J Pharmacol Exp Ther 2007;323(1):202–209. doi: 10.1124/jpet.107.125757
[49] Nitti VW, Auerbach S, Martin N et al. Results of a randomised phase III trial of mirabegron in patients with overactive bladder. J Urol 2013;189(4):1388–1395. doi: 10.1016/j.juro.2012.10.017
[50] Chapple CR, Kaplan SA, Mitcheson D et al. Randomised double-blind, active-controlled phase III study to assess 12 month safety and efficacy of mirabegron, a beta 3 adrenceptor agonist, in overactive bladder. Eur Urol 2013;63(2): 296–305. doi: 10.1016/j.eururo.2012.10.048
[51] Drake MJ, Chapple C, Esen AA et al.; BESIDE investigators. Effiacacy and safety of mirabegron add-on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4-week solifenacin monotherapy: a randomised double blind muticentre phase 3B study (BESIDE). Eur Urol 2016;70(1):136–145. doi: 10.1016/j.eururo.2016.02.030
[52] Herschorn S, Chapple CR, Abrams P et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int 2017;120(4):562–575. doi: 10.1111/bju.13882
[53] Robinson D, Kelleher C, Staskin D et al. Patient reported outcomes from SYNERGY, a randomised double blind multicentre study evaluating combinations of mirabegron and solifenacin compared with monotherapy and placebo in OAB patients. Neurourol Urodyn 2018;37(1):394–406. doi: 10.1002/nau.23315
[54] Hong SK, Yang JH, Kim TB, Kim SW & Paick JS. Effects of ovariectomy and oestrogen replacement on the function and expression of Rho-kinase in rat bladder smooth muscle. BJU Int 2006;98(5):1114–1117. doi: 10.1111/j.1464-410X.2006.06486.x
[55] Yoshida J, Aikawa K, Yoshimura Y et al. The effects of ovariectomy and oestrogen replacement on acetylcholine release from nerve fibres and passive stretch induced acetylcholine release in female rat bladder. Neurourol Urodyn 2007;26(7):1050–1055. doi: 10.1002/nau.20438
[56] Cardozo L, Lose G, McClish D & Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta Obstet Gynecol Scand 2004;83(10):892–897. PMID: 15453881
[57] Tseng LH, Wang AC, Chang YL et al. Randomized comparison of tolterodine with vaginal estrogen cream versus tolterodine alone for the treatment of postmenopausal women with overactive bladder syndrome. Neurourol Urodyn 2009;28(1):47–51. doi: 10.1002/nau.20583
[58] Nelken RS, Ozel BZ, Leegant AR, Felix JC & Mishell DR. Randomised trial of oestradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause 2011;18(9):962–966. doi: 10.1097/gme.0b013e3182104977
[59] Jiang F, Zhu L, Xu T et al. Efficacy and safety of solifenacin succinate tablets versus solifenacin succinate tablets with local oestrogen for the treatment of overactive bladder in postmenopausal women – a multicentre, randomised, open label, controlled comparison study. Menopause 2016;23(4):451–457. PMID: 26757270
[60] Chughtai B, Forde JC, Buck J et al. The concomitant use of fesoterodine and topical vaginal oestrogen in the management of overactive bladder and sexual dysfunction in postmenopausal women. Post Reprod Health 2016;22(1):34–40. doi: 10.1177/2053369116633017
[61] Serati M, Salvatore S, Uccella S, Cardozo L & Bolis P. Is there a synergistic effect of topical oestrogens when administered with antimuscarinics in the treatment of symptomatic detrusor overactivity? Eur Urol 2009;55(3):713–719. doi: 10.1016/j.eururo.2008.06.051
[62] Norgaard JP, Rittig S & Djurhuus JC. Nocturnal enuresis: an approach to treatment based on pathogenesis. J Pediatr 1989;114(4 Pt 2):705–710. PMID: 2926585
[63] Mattiasson A, Abrams P, Van Kerrebroeck P, Walter S & Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double blind placebo controlled studying men. BJU Int 2002;89(9):855–862. PMID: 12010228
[64] Robinson D, Cardozo L, Akeson M et al. Women take control; Desmopressin – a drug for daytime urinary incontinence. Neurourol Urodyn 2002;21(4):385–386. doi: 10.1111/j.1464-410X.2004.04768.x
[65] British National Formulary. Desmopressin. Available at: https://bnf.nice.org.uk/drug/desmopressin.html (accessed March 2019)
[66] Nocdurna product information; Ferring Pharmaceuticals. Available at: https://www.nocdurna.com/hcp/ (accessed March 2019)
[67] Binz T, Sikorra S & Mahrhold S. Clostridial neurotoxins: mechanism of SNARE cleavage and outlook on potential substrate specificity reengineering. Toxins (Basel) 2010;2(4):665–682. doi: 10.3390/toxins2040665
[68] Tincello DG, Kenyon S, Abrams KR et al. Botulinum toxin A versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol 2012;62(3):507–514. doi: 10.1016/j.eururo.2011.12.056
[69] Peters KM, Macdiarmid SA, Wooldridge LS et al. Randomised trial of percutaneous tibial nerve stimulation versus extended release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 2009;182(3):1055–1061. doi: 10.1016/j.juro.2009.05.045
[70] Burton C, Sajja A & Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn 2012;31(8):1206–1216. doi: 10.1002/nau.22251
[71] van der Pal F, van Balken MR, Heesakkers JP et al. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU Int 2006;97(3):547–550. doi: 10.1111/j.1464-410X.2006.06055.x
[72] Peters KM, Carrico DJ, Macdiarmid SA et al. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24-month results of the STEP study. Neurourol Urodyn 2013;32(1):24–29. doi: 10.1002/nau.22266
[73] Oerlemans DJ & van Kerrebroeck PE. Sacral nerve stimulation for neuromodulation of the lower urinary tract. Neurourol Urodyn 2008;27:28–33. doi: 10.1002/nau.20459
[74] Schmidt RA, Senn E & Tanagho EA. Functional evaluation of sacral nerve root integrity. Report of a technique. Urology 1990;35(5):388–392. PMID: 2336766
[75] Monga AK, Tracey MR & Subbaroyan J. A systematic review of clinical studies of electrical stimulation for treatment of lower urinary tract dysfunction. Int Urogynecol J 2012;23(8):993–1005. doi: 10.1007/s00192-012-1691-5
[76] Groen J, Blok BF & Bosch JL. Sacral neuromodulation as treatment for refractory idiopathic urge urinary incontinence: 5-year results of a longitudinal study in 60 women. J Urol 2011;186(3):954–959. doi: 10.1016/j.juro.2011.04.059
[77] Nitti VW, Dmochowski R, Herschorn S et al.; Embark Study Group. Onabotulinumtoxin A for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013;189(6):2186–2193. doi: 10.1016/j.juro.2012.12.022
[78] Burton C, Saija A & Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and metanalysis. Neurourol Urodyn 2012;31(8):1206–1216. doi: 10.1002/nau.22251
[79] Siegel S, Noblett K, Mangel J et al. Three year follow up results of a prospective multicentre study in overactive bladder patients treated with sacral neuromodulation. Urology 2016;94:57–63. doi: 10.1016/j.urology.2016.04.024