age fotostock / Alamy
In this article you will learn:
- The potential risks of mixing medicines
- Common incompatibility reactions in practice
- How to prevent problems when mixing medicines
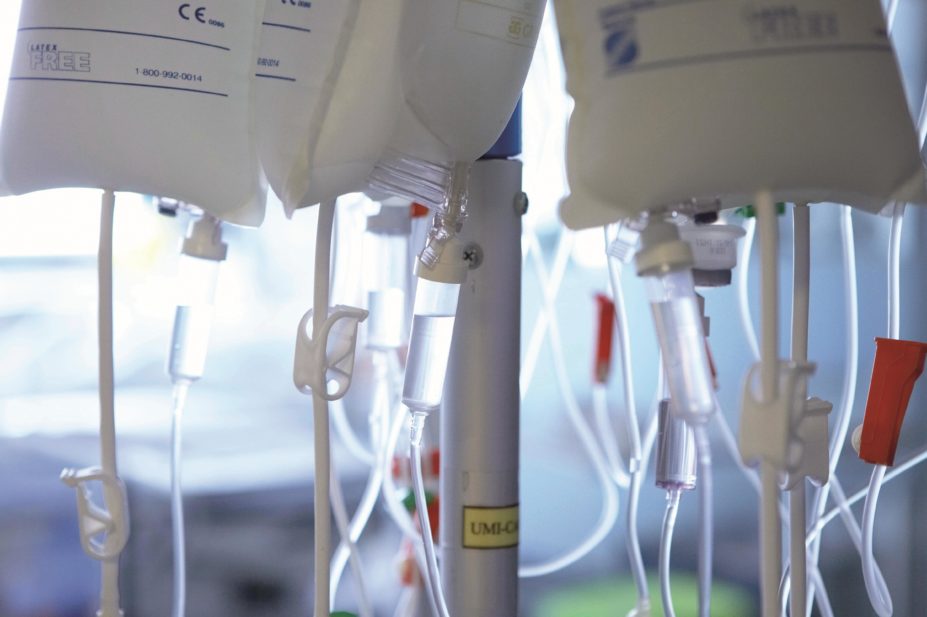
Healthcare professionals are often required to mix medicines together before administration to a patient. In the UK, this is permissible when one of the products is used as a vehicle for the administration of another (see ‘Guidance on mixing in the UK’). However, there are situations when there is a clinical need to mix medicines, such as when a patient is on multiple drug infusions and there is limited parenteral access, the patient has been placed on fluid restriction in palliative care, and when there is an urgent clinical need for multiple medicines to be administered within a short space of time.
Mixing must only be undertaken when it is essential to meet the needs of the patient and should never be performed at the convenience of the healthcare professional[1]
.
There is limited information available on the incidence of serious events caused by drug incompatibilities and no major incidents have ever been reported through the NHS England National Reporting and Learning System. However, this lack of reporting may be because the adverse effects caused by drug incompatibilities are difficult to identify in patients who are already seriously ill[2]
,[3]
.
Guidance on mixing in the UK
The legal position with regards to mixing parenteral injections in clinical practice in the UK is documented in guidance from the Department of Health. This guidance explains which healthcare professionals can prescribe and administer medicines that are intended to be mixed. It also stipulates that the mixing of medicines should:
- only be undertaken in the best interests of the patient;
- be avoided where possible;
- only be done by a person who is competent and willing to do so;
- take place in a pharmacy (where possible).
Those prescribing medicines intended to be mixed should provide instructions on how to do so in writing and must satisfy themselves that the persons doing the mixing are competent to undertake the task safely and effectively. The Department of Health advises prescribers and those administering injections to check with a pharmacist, or other authoritative source, about whether it is safe to mix injectable medicines[1]
.
Risks of mixing
When two or more medicines and their diluents are mixed together in the same syringe or infusion bag, or when two or more infusion lines meet at a Y-site junction, there is a risk that they may be incompatible with one another. The type of infusion vehicle used for administration of the drug can also affect the stability of the drug. For instance, oxaliplatin can be stable in glucose 5%; however, when mixed with any chloride ion-containing infusion, such as sodium chloride, it will degrade rapidly to form impurities. As a result, the clinical consequences of inappropriate mixing can cause severe harm to a patient.
Physicochemical reactions within the mixture can cause precipitation, separation, gas formation and changes in colour or turbidity. This can, theoretically, result in catheter occlusion or emboli formation, causing organ failure and death. Typically, these reactions are detectable through a visual check.
Chemical instability between drugs can lead to reduced effectiveness of one or all of the active ingredients, causing therapeutic failure or the production of toxic compounds. This effect is often not visible, and a clear, colourless fluid free of precipitation is not definitive proof of compatibility between two or more substances[2]
,[3]
.
Although the causes of incompatibility reactions are mostly as a result of active drug reactions, occasionally incompatibility between the excipients in various parenteral presentations may also occur.

Source: John Bentley
1) Propofol and lidocaine incompatibility, resulting in layering and coalescence of propofol’s vehicle
2) Phenytoin precipitation caused by mixing with a solution that lowers its pH (e.g. glucose 5%)
Examples of incompatible drug reactions
| Incompatible drugs | Reason for incompatibility | Possible action to take |
|---|---|---|
Calcium-containing additives and phosphate-containing additives in total parenteral nutrition (TPN)
| Concentration related — above certain concentrations for either or both additives, an insoluble additive is formed | Reduce concentrations where possible; do not add calcium and phosphate one after the other to the TPN bag being prepared |
Oxycodone and cyclizine in syringe drivers
| Concentration-related precipitation above 3mg/ml of cyclizine | Use water for injection as the diluent instead of sodium chloride 0.9%; consider alternative analgesics such as alfentanil |
Amphotericin and electrolyte solutions
| Ionic incompatibilities; amphotericin can precipitate out | Avoid electrolyte solutions (e.g. sodium chloride); use glucose 5% infusions where possible, and solutions which have a pH > 4.2 |
Furosemide and low pH solutions
| Low pH solutions | Potentially precipitates out in low pH solutions (e.g. glucose 5% solutions); use sodium chloride 0.9% where possible |
Atracurium and high pH solutions
| High pH solutions | Incompatible with high pH solutions, must be administered separately to drugs with high pH such as thiopental to prevent precipitation |
Lidocaine and propofol
| Layering and coalescence of propofol vehicle, caused by lidocaine disrupting the surfactant properties of the vehicle | Use low lidocaine concentrations or administer lidocaine and propofol separately |
Physicochemical reactions
Changes in pH can cause drugs to precipitate out of solution. Most medicines are small chemical organic molecules that are often formulated as weak acid or weak base salts. Formulation in this manner, at a pH where the drug is predominantly in its ionised form, increases the solubility of the drug when added to an aqueous solution for administration. The pH of the administration fluid is important because it determines whether the drug will be in an ionised or unionised form when it is diluted[4]
.
Typically, the buffer capacity of the diluent solution will allow it to resist any small changes to the pH when the drug is added to the infusion bag or other vehicle for administration. However, if the pH of the drug is significantly altered by the fluid to which it is added or by the addition of another drug, a precipitate of unionised, insoluble drug may form. For this reason, acid-base reactions are the most common cause of precipitation from incompatible drug-drug or drug-diluent combinations. The influence that a fluid or second drug can have on the pH of a solution depends on: the quantity being added, the difference in pH between drugs or drug and diluent and the buffering capacity of the solution[5]
.
A common example of pH affecting stability is with morphine sulphate. This is a weak base, but is formulated at an acidic pH of 2.5–6.5. It is highly sensitive to changes in pH, and likely to precipitate out of solution in an alkaline environment.
Phenytoin is a weak acid and is formulated for use in intravenous preparations as a phenytoin sodium salt, with the solution adjusted to a highly basic pH of 12. Phenytoin can precipitate if mixed with a solution that lowers its pH; for example, when mixed with glucose 5% (pH range 3.2–6.5), phenytoin precipitates almost immediately.
According to its manufacturer, phenytoin sodium can be added to sodium chloride for infusion, but administration should start immediately after preparation and must be completed within one hour. The infusion solution should be constantly monitored for haziness and precipitate. An in-line filter should also be used to prevent any precipitate entering a patient’s blood.
| Table 1. pH of regularly used infusion fluids | ||
|---|---|---|
| Source: American Society of Health-System Pharmacists[6] | ||
Solution | pH range | Buffer capacity |
Sodium chloride 0.9% | 4.5–7.0 | Low |
Glucose 5% | 3.2–6.5 | Low |
Sodium chloride 0.45% or 0.9% with glucose 5% | 3.2–6.5 | Low |
Compound lactate (Hartmann’s solution) | 5.0–7.0 | High |
Ionic reactions
, o
r “salting out”, occurs when ionic bonds form between two opposing ions in solution. Valency relates to the number of electrons available for bonding in an atom’s outermost shell, determining the charge that the ion can hold; salts containing polyvalent anions and cations (e.g. calcium, magnesium or sulphate) can form stronger bonds, and are generally less soluble than salts in which one or both of the ions is monovalent (e.g. sodium, chloride, potassium or a
cetate)
[4]
.
Although drugs are generally formulated as salts using monovalent ions, they can form insoluble salts if they are mixed with products containing polyvalent ions (e.g. total parenteral nutrition [TPN], Hartmann’s solution, calcium chloride or magnesium sulphate). For example, ceftriaxone is formulated as a disodium salt, but when mixed with calcium-containing solutions can form a highly insoluble ceftriaxone calcium salt.
Changes in concentration can affect the compatibility of some drug combinations (i.e. drugs that are compatible at lower concentrations can become incompatible at higher concentrations)[7]
. This can be a result of an increased rate of reaction between two compounds forming an insoluble compound or if the solute concentration becomes too high. For example, when calcium and phosphate additives are added to a TPN bag there is a potential for an insoluble calcium phosphate salt to be formed above too high a concentration for either or both additives. Different amino acids in the TPN solution can also affect the concentration at which calcium phosphate is formed. Concentration is particularly important when multiple drugs are being infused subcutaneously via a syringe driver, in which minimal volumes are required.
Alcohol or lipid solvents are water-miscible organic solvents used to improve the solubility of poorly water soluble drugs. These are required not only to achieve high solubility but also to prevent drug precipitation upon dilution in an aqueous solution. Commonly used solvents include polyethylene glycol, propylene glycol, ethanol and glycerine[9]
.
When the formulated product is diluted in an aqueous solution, the drug may precipitate out until enough solution is added to enable dissolution without the need for a solvent. Therefore, it is often impracticable to mix drugs that are poorly water soluble with drugs that are highly water soluble. The solubility of the drug and use of an alcohol or lipid solvent can also determine how, and in what vehicle, the drug should be administered.
For example, digoxin is formulated with propylene glycol 40% and ethanol 10%. It needs to be administered diluted at least fourfold to prevent precipitation.
Diazepam is insoluble in aqueous solutions and is formulated as an emulsion (e.g. Diazemuls) or in a high alcohol-containing solvent. Diazemuls can be mixed in all proportions with Intralipid 10% or 20% (a lipid emulsion, particularly useful in providing a vehicle for drugs such as diazepam to be administered), but not with saline solutions. According to the manufacturers, the alcohol-containing formulation should be diluted to a concentration of at least 10mg in 200ml. There is some evidence that higher concentrations may be stable, but these are not recommended[6]
.
The creation of gas is a normal part of the reconstitution of some drugs, particularly those made with sodium carbonate as an excipient (upon reconstitution, these formulations can generate carbon dioxide). However, the creation of extensive amounts of gas can be hazardous. Metoclopramide formulated as its hydrochloride salt is incompatible with sodium bicarbonate for this reason[6]
.
Fat emulsions, such as Intralipid, TPN and some drug formulations (e.g. Diazemuls), can easily be destabilised, cracked or separated when they are mixed with solutions containing highly positively charged ions. For this reason, electrolytes should only ever be added to TPN in a specialist pharmacy aseptic unit where the stability of the final product can be checked before use.
Propofol is formulated as a lipid. When it is mixed with too much lidocaine, the fat droplet size increases considerably and layering occurs (fat and aqueous components of an emulsion separate over time into two distinct layers). This is caused by lidocaine disrupting the surfactant properties of the propofol vehicle[6]
.
Chemical instability
Oxidation, reduction and hydrolysis can cause the chemical degradation of a drug, resulting in a loss of potency or the formation of toxic by-products. These processes can be accelerated by: the addition of an aqueous solution when reconstituting dry powders; ultraviolet light; exposure to oxygen; and changes in temperature or pH.
When erythromycin is reconstituted, it is degraded by hydrolysis and will lose potency after eight hours. This reaction is accelerated by a change in pH[6]
and therefore erythromycin should not be diluted with glucose or combined with acidic drugs, such as linezolid.
Adsorption— when a drug in solution binds to the surface of its container, giving set or in-line filter — reduces the amount of drug available to be administered to the patient. Large polymeric drugs (e.g. paclitaxel), proteins (e.g. insulin) and highly lipid-soluble drugs, the flow rate, concentration of drug and pH can all affect the extent to which adsorption occurs.
In-line filters are required for certain medicines (e.g. phenytoin, TPN and monoclonal antibodies); this may preclude the mixing of other drugs in the same infusion. If an in-line filter becomes blocked and the fluid is prevented from running, a new infusion should be prepared as there could still be precipitate or particles in the infusion bag.
Adsorption is also more likely to occur when polyvinyl chloride (PVC)-containing equipment is used. Syringes are generally PVC-free and are more suitable for use with drugs that are susceptible to adsorption. PVC-free giving sets should be obtained where possible[8]
.
Denaturation can occur when biological compounds — large, complex molecules that are extremely sensitive to being denatured — are mixed with other drugs, particularly if they are exposed to extremes of pH, or if they are agitated for a prolonged period. Biologics, such as blood products and monoclonal antibodies, should never be mixed with other drug compounds and should always be administered through separate lines.
Reducing risk
A number of factors should be considered before mixing medicines to reduce the risk of inappropriate mixing.
Avoid mixing by using an alternative route of drug administration (e.g. rectal or sublingual), or prescribing a suitable alternative drug. Multi-lumen tube sets can also be used to allow a number of different intravenous drugs to be administered separately at the same time.
Staggering the administration times of medicines or determining if one medicine can be temporarily stopped, without compromising patient care, while another is given, can also prevent the need to mix medicines.
When intravenous drugs are not mixed but are given consecutively, the infusion line should be flushed with compatible fluid between each administration[9]
.
Monitor for reactions caused by drug incompatibilities, even when mixing apparently compatible medicines. Infusion bags, syringes and site of mixing should be frequently inspected for any signs of incompatibility, such as precipitation. Patients should be monitored for injection site reactions, such as thrombophlebitis, and signs of organ failure. Since chemical reactions between the mixed drugs can result in a reduction in the amount of active drug, patients should also be monitored for treatment failure.
Seek advice about the compatibilities of the drug that is to be mixed. Information provided by the manufacturer should always be consulted first. Section 6.6 of a drug’s Summary of Product Characteristics provides information about the potential incompatibilities of admixtures of the drug in question. Reference sources, such as the American Society of Health-System Pharmacists’s ‘Handbook on injectable drugs’, can be used to determine which medicines and infusion fluids are compatible[6]
. The online Medusa database for injectables is also a useful resource for injectable drug monographs. Finally, medicines information centres can provide advice about mixing injectable medicines[1]
. Compatibility data are, however, limited and are often restricted to certain diluents and concentrations.
John Bentley is a surgical pharmacist, Katie Heard is a resident pharmacist and Chris Chung is lead directorate pharmacist for imaging and anaesthetics, all at Chelsea and Westminster Hospital NHS Foundation Trust. George Collins is lead quality assurance pharmacist at East Kent Hospitals University NHS Foundation Trust.
References
[1] Department of Health. Mixing of medicines prior to administration in clinical practice: medical and non-medical prescribing. London: DH; 2010.
[2] Tissot E, Cornette C, Demoly P et al. Medication errors at the administration stage in an intensive care unit. Intensive Care Med 1999;25:353–359.
[3] Bertsche T, Mayer Y, Stahl R et al. Prevention of intravenous drug incompatibilities in an intensive care unit. Am J Health Syst Pharm 2008;65:1834–1840.
[4] Newton D. Drug incompatibility chemistry. Am J Health Syst Pharm 2009;66(4):348–357.
[5] Alvarez-Núñez FA & Yalkowsky SH. Buffer capacity and precipitation control of pH solubilized phenytoin formulations. Int J Pharmaceutics 1999;185(1):45–49.
[6] Trissel LA. Handbook on injectable drugs. 17th edition. Bethesda: American Society of Health-System Pharmacists; 2007.
[7] Dickman A & Schneider J. The Syringe Driver. Third edition. Oxford: University Press 2011.
[8] Pharmacy Department. University College London Hospitals. UCL Hospitals Injectable Medicines Administration Guide. Third edition. London: Wiley-Blackwell; 2011.
[9] Royal College of Nursing. Standards for infusion therapy. London: RCN; 2010.


