Jonathan Pickard
Ancient, potent, effective; opioids are an enduring mainstay in the management of severe pain, yet one that has been subjected to mounting scrutiny[1]
. There is increasing evidence of long-term adverse effects from opioid use, coupled with controversy over the long-term efficacy of these medicines[2],[3]
. Despite the prevalent impetus this has created to de-prescribe opioids in the context of chronic pain, their steadfast role in the palliative patient demographic makes it essential that pharmacists and healthcare professionals remain fully conversant with their use.
This article provides an overview of applicable and practical knowledge on the pharmacology and clinical use of opioid analgesics in those with life-limiting illness, to empower and support prescribers in the current environment of waning familiarity with these medicines. It also outlines how pharmacists may select, initiate, titrate, switch and optimise these medicines in a palliative care context, as well as the factors relevant to opioid use in the more immediate end-of-life period. Reference will be made to National Institute for Health and Care Excellence (NICE) guidance and the British National Formulary, alongside presenting the evidence underpinning guideline recommendations. In addition, the principal importance of prescribers following local implementation of this guidance, and in arriving at shared decisions with patients, is highlighted.
What ‘palliative’ means
The World Health Organization (WHO) uses the term ‘palliative care’ to describe an approach to improving the quality of life of patients facing life-threatening illness through management of symptoms, such as pain[4]
. Life-threatening illnesses may be chronic, meaning a palliative care approach may span years and operate in conjunction with life-prolonging therapies, right up until end of life (or recovery). Palliative care encompasses end-of-life care, which broadly refers to care given within the last year of life, so palliative and end-of-life care are not synonymous[5]
. Furthermore, a patient’s journey with a palliative diagnosis traverses a shifting landscape: pathologies alter and evolve; pain control needs are dynamic; and patient preference and practicality must guide the prescriber. For example, pain control needs and medicine administration methods will differ when facing the waning consciousness or diminishing swallow of an individual in their last hours to days of life, compared with someone whose prognosis may span many active months. Diversity in patients’ journeys must give rise to diversity in prescribing approaches. This article concentrates on managing adults considered to be in the last year of life in the context of cancer pain, since use of strong opioids for chronic non-cancer pain is associated with lesser benefit and greater risk[3]
. Indeed, risk and benefit must still be carefully balanced, even when opioids are used within this last year of life — a 2017 Cochrane review found that the absolute adverse event rate for opioids used in patients with chronic non-cancer pain between 2 weeks and 13 months was 78% compared with placebo, with an absolute serious adverse event rate of 7.5% when compared with placebo[2]
.
The analgesic ladder and guiding principles
In 1986, WHO proposed a three-step analgesic ladder for managing cancer pain, beginning with a non-opioid (with or without an adjuvant) analgesic as step 1, escalating to a weak opioid (with or without an adjuvant, with or without a non-opioid) as step 2 if pain persists or worsens and, finally, advocating a strong opioid in moderate-to-severe pain as step 3 (with or without an adjuvant, with or without a non-opioid)[4]
. Regular administration of oral analgesic preparations — titrated according to response and benefit — in individuals who are monitored and managed for adverse effects, and provided with additional analgesia on an ‘as required’ basis, represent the guiding principles of cancer pain management[6]
.
While the WHO’s tenets remained largely unchanged in the intervening decades, randomised trial evidence now calls into question the utility of step 2[7]
. Bandieri et al. conclude that low-dose step 3 analgesia (morphine) offers a more rapid and significant reduction in pain intensity compared with step 2 weak opioids, with a similar tolerability, when used in moderate cancer pain[7]
. Therefore, escalating from a non-opioid to a low-dose strong opioid is considered a preferable option (see Figure)[7],[8]
. Unlike strong opioids, another disadvantage of step 2 weak opioids is the analgesic ‘ceiling’ effect — where dose escalation beyond a certain level does not confer any additional analgesic benefit[8]
. Moreover, most of the analgesic effect of the weak opioid codeine is gathered from its O-demethylation into morphine[9]
. The extent of this conversion is governed by genetic polymorphism and so is subject to wide inter-individual variation, which results in equally variable responses in analgesic efficacy[9]
. Codeine may be considered a pro-drug of morphine (i.e. once taken, it is metabolised into a pharmacologically active drug); however, it is metabolised inconsistently to yield < 10% morphine[10]
. Therefore, a simpler solution is to avoid this unpredictability by using low-dose morphine as a reliably quantifiable alternative to an outdated step 2 opioid.
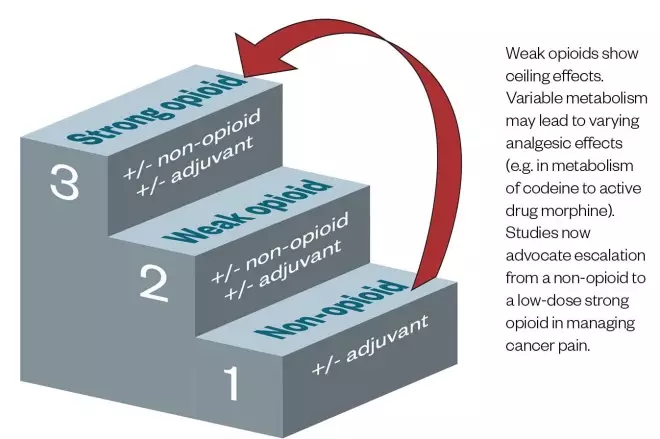
Figure: Potential solution to the 1986 World Health Organization Pain Ladder[7],[8]
Source: World Health Organization[4]
However, despite the familiarity with, and preference for, morphine as a go-to strong opioid, pharmacists and prescribers must remember a choice exists. Having now established the role for strong opioids, the question turns to which strong opioid should be chosen.
Choosing the right opioid
Opioid metabolism and clearance considerations
Opioids, as a drug class, are lipophilic. This is essential for penetration of the central nervous system and mediation of therapeutic effects[11]
. For most opioids, the major route of excretion is through the kidneys; however, for this to occur, hepatic metabolism is required to produce derivatives with greater water solubility[12],[13]
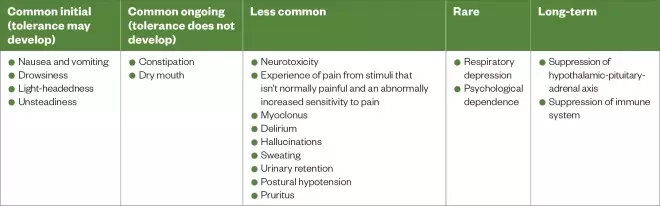
. Therefore, impairment in one or both of these organs can significantly reduce opioid clearance and lead to accumulation. Although the opioid class has a range of side effects (see Table 2), these may be amplified by this process[11]
.
In the context of renal impairment, the pharmacological activity of hepatic metabolites must be considered (see Table 1)[12],[13],[14],[15],[16],[17],[18],[19],[20]
. Morphine undergoes glucuronidation to form morphine-6-glucuronide (M6G), which is pharmacologically active at the mu opioid receptor that mediates analgesia, hypothermia, respiratory depression, bradycardia, nausea, euphoria and physical dependence[14],[15]
. Importantly, M6G relies on renal excretion[15]
. Unsurprisingly, the half-life (i.e. the time for 50% of a drug/metabolite to be removed from the body) of M6G can increase significantly with renal impairment, with an increase from 3–5 hours to upwards of 50 hours possible in severe impairment[13]
. Therefore, morphine should be avoided in patients with significant renal dysfunction owing to the risk of accumulation and the potential for prolonged toxicity. In contrast, alfentanil is metabolised by oxidation to inactive metabolites[13],[16]
. Although these by-products can accumulate in renal impairment, they do not have any pharmacological activity and, consequently, alfentanil can be regarded as safe in such circumstances
[13].
With regard to hepatic impairment, the type of metabolic processes required for modification of the individual opioid should be considered (see Table 1)[12],[13],[14],[15],[16],[17],[18],[19],[20]
. Such processes can be broadly classified as phase 1 modification reactions (e.g. oxidation) and phase 2 conjugation reactions (e.g. glucuronidation)[12]
. As hepatic impairment advances, drug metabolism diminishes and accumulation potentially results. Notably, with advancing impairment, phase 1 reactions are reduced to a greater extent than their phase 2 counterparts[12],[17]
. Morphine, as previously discussed, is metabolised by glucuronidation — a phase 2 reaction[14],[15]
. In severe hepatic impairment, glucuronidation of morphine can still occur, although to a lesser extent[18]
. Consequently, morphine can be used, albeit cautiously, with gradual dose titration and increased dosing intervals, in patients with cirrhosis (see Table 1)[12],[13],[14],[15],[16],[17],[18],[19],[20]
. By comparison, the major metabolic route for oxycodone is N-dealkylation (a phase 1 reaction), which can be substantially impaired in severe cirrhosis, leading to an almost four-fold increase in half-life[19]
[20]
. Given the risk of accumulation, oxycodone should be avoided in patients with hepatic cirrhosis.
Both hepatic and renal impairment can influence the pharmacokinetics of opioids beyond a simple reduction in metabolism and excretion, for example:
- Distribution — reduced protein binding (secondary to hypoalbuminaemia);
- Pharmacodynamic effects — such as increased cerebral sensitivity[12],[13],[17]
.
Specialist advice should be sought in patients with impairment of both hepatic and renal function.
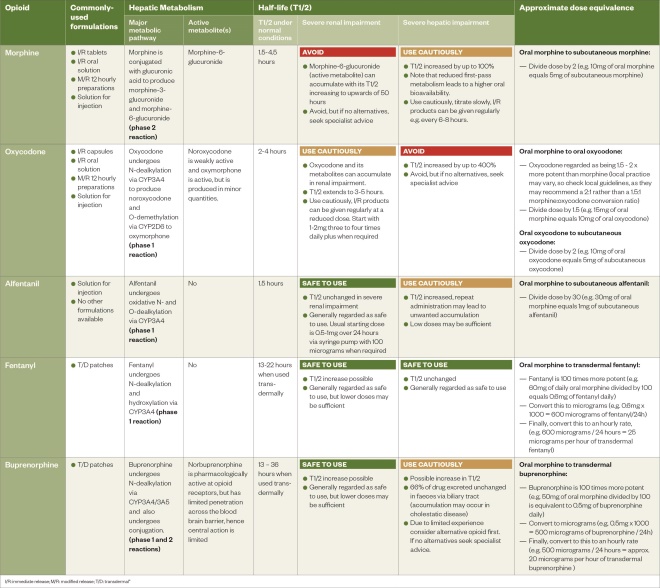
Table 1: Overview of opioids (Click here for full-size PDF version)
Route of administration
A preferred or practical route of administration can influence selection, with oral formulations (see Table 1) being recommended[5],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21]
. Injections may be appropriate at the end of life, but in individuals with stable pain and enduring enteral absorption issues (e.g. short bowel syndrome), transdermal preparations may be more acceptable[22],[23]
.
Interactions
Opioids are metabolised by cytochrome P450 (CYP450) enzymes and are consequently subject to drug-drug interactions mediated by enzyme induction or inhibition (see Table 2)[24]
. When selecting an opioid for initiation, the presence of interacting drugs should be considered. For example, clarithromycin can increase serum levels of oxycodone through inhibition of CYP3A4; therefore, initiating oxycodone when a patient is receiving clarithromycin risks elevated serum levels and causing adverse effects[25]
. Once the antibiotic course has finished, serum levels may decline and pain may be precipitated. Such variation in serum opioid levels may be difficult to manage, particularly in an outpatient setting. Therefore, it may be prudent to choose another opioid that does not interact (e.g. morphine).
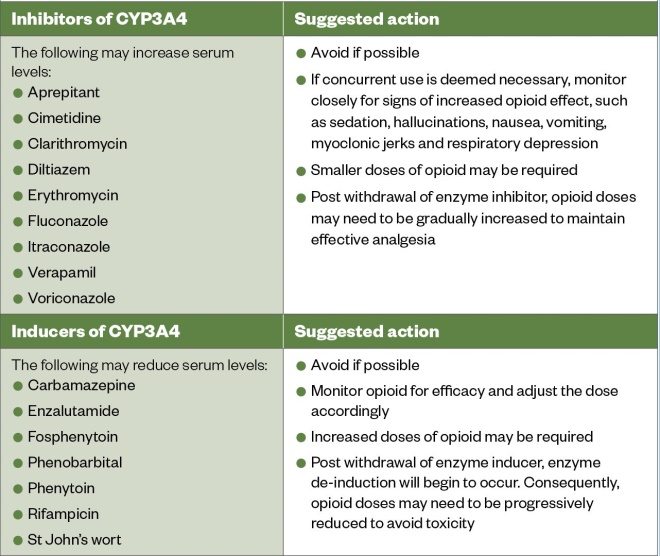
Table 3: Cytochrome P3A4 enzyme inducers and inhibitors that may significantly interact with the opioids alfentanil, buprenorphine, fentanyl and oxycodone
Source: Stockley’s Drug Interactions
[24]
How to initiate oral strong opioids
When initiating opioid naïve patients with advanced and progressive disease, NICE guidance recommends two options — starting either regular oral modified-release morphine or regular oral immediate-release morphine[21]
. A third option involves rotating from a weak opioid. Regardless of the option selected, a noteworthy adjunct to each of these regimes is the availability of additional analgesia on an ‘as required’ basis.
Where oral administration is not suitable, but pain is stable, NICE recommends a transdermal opioid patch. Where the oral route is unsuitable and the pain is unstable, subcutaneous opioid delivery is recommended.[21]
[22]
In either of these cases, specialist support may be required[21]
.
As NICE guidance recommends oral morphine as the first-choice strong opioid, the focus of this article will be on three methods of initiating a patient on opioids via the oral route[21]
.
Rotating from a weak opioid
Weak opioids show ceiling effects. Moreover, both codeine and tramadol are prone to variations in metabolism. For example, the inconsistent metabolism of codeine via CYP450 2D6 can render it useless in poor metabolisers, while putting ultra-rapid metabolisers at risk of toxicity[6]
. When metabolised, codeine has been shown to yield around 10% of the active analgesic morphine, and tramadol (as a codeine derivative) is considered comparable to codeine in its analgesic efficacy[10],[26]
. Therefore, when rotating to morphine from a weak opioid it is possible to approximate an equivalent morphine dose.
For example, a patient has been taking 100mg tramadol four times per day (equal to 400mg/24 hours) for a week. As tramadol is around one-tenth as potent as morphine, this equates to 40mg oral morphine/24 hours. For patients taking the maximum dose of tramadol (400mg/24 hours) and still experiencing pain, conversion to a strong opioid to allow further titration can be done in one of two ways:
- 10mg morphine sulphate four times per day during waking hours, with 10mg morphine sulphate on an ‘as required’ basis (hourly); or
- 20–30mg modified-release morphine sulphate twice daily with immediate-release morphine sulphate on an ‘as required’ basis (hourly) at a dose equivalent to between one-sixth and one-tenth of the total daily morphine dose (e.g. between 5mg and 10mg)[27]
.
These and all other conversion ratios described are approximations only. Variation in consensus exists, so these conversions are to be used in the context of clinical judgement and with reference to local guidelines. When converting between strong opioids at higher doses, such variations are magnified and both caution and specialist advice are needed. In addition, owing to the serotonergic and noradrenergic effects of tramadol, patients taking this medicine long-term require specialist advice to avoid withdrawal when rotating[8]
. This is not a consideration in this example, as the patient has been taking the medicine for one week only.
Starting with low-dose oral modified-release opioids
In this method, modified-release morphine is commenced at a low dose (e.g. 10–15mg twice daily) – 10mg modified-release morphine twice daily would generally be the starting dose in older or frail people[21],[28]
. This is given alongside immediate-release morphine on an ‘as required’ basis for breakthrough pain at a dose one-sixth to one-tenth of the total 24-hour morphine dose[28]
.
For example, an opioid-naïve patient is commenced on 10mg twice daily morphine. This equates to 20mg/24 hours. One-sixth of this dose is 3.3mg and one-tenth is 2mg. Thus, a breakthrough dose of 2.5mg immediate-release morphine solution sits within this range and is prescribed on a when required, hourly basis.
This method is simpler than initiating morphine using an immediate-release preparation, but its disadvantages lie in circumventing the fine and rapid titration phase offered by the latter[29]
. For example, titration of modified-release preparations should be undertaken every two to three days, meaning that this method lacks the day-to-day responsiveness of an immediate initiation regime[28]
. In practical terms, patients may prefer the simplicity of twice daily opioid administration, which must be taken into account when selecting an initiation regime.
Starting an immediate-release opioid preparation
Immediate-release morphine can also be initiated in an opioid-naïve patient using a regular immediate-release preparation and an ‘as required’ dose (e.g. BNF and NICE guidance recommend starting at 20–30mg in divided doses, which could, for example, be given as 5mg morphine sulphate immediate-release solution every four hours with 5mg immediate-release morphine sulphate when required for breakthrough pain)[21],[27],[30]
. Indeed, this method of initiation is recommended in clinical practice by the European Society for Medical Oncology[6]
.
Immediate-release preparations of morphine used in this manner confer advantages including:
- Onset of action is rapid (peak plasma concentrations achieved within an hour);
- Action is ephemeral (analgesia lasts around four hours) – meaning that fine, responsive titration according to a patient’s pain can be made and steady state (i.e. amount of a drug being absorbed is the same amount that is being cleared from the body during therapy) achieved within 24 hours
[30]
.
Clinically, in moderate-to-severe cancer pain, this translates into a median time-to-pain control of eight hours, with 79% of patients in the MERITO study by De Conno et al. initiated on a regime of 5-10mg immediate-release morphine every four hours, experiencing at least a 50% reduction in pain intensity within 24 hours[29]
. Modified-release morphine preparations, by contrast, have an analgesic effect that lasts 12 hours, and the drug reaches an attenuated peak plasma concentration 2–4 hours after ingestion[30]
. These characteristics prevent rapid dose adjustment and fine dose titration[30]
.
After one to two days of immediate-release morphine (given as a regular four-hourly dose on an ‘as required’ basis), a new dose should be calculated by totalling up all of the morphine used in the previous 24 hours and dividing by six[28]
. An important caveat to this is that, proportionally, opioid doses should not be escalated by more than 33–50% in 24 hours[27],[28],[31]
. NICE indicates that a regular dose can be titrated until a balance exists between acceptable pain control and side effects[21]
. Alternatively, once a stable four-hourly dose is attained, the patient may be transitioned to a 12-hour modified-release preparation — where the 12-hour dose will be half of the total regular morphine dose received in the previous 24 hours[31]
. When required, immediate-release morphine should remain available at a dose one-sixth to one-tenth of the total daily modified-release morphine dose[28]
.
For example, an opioid-naïve patient commences 5mg immediate-release morphine sulphate every four hours during waking hours, with immediate-release 5mg morphine hourly (when required) for breakthrough pain. During the first two days of treatment they consistently require 4 x 5mg immediate-release morphine, in addition to their regular dose of immediate-release morphine sulphate. Pain is effectively controlled on this regime.
Their total 24-hour morphine dose both days is therefore 40mg (‘as required’ dose plus regular dose).
The prescriber has the option to change the dose as follows:
- Increase the dose to 10mg every four hours during waking hours, with 10mg when required; or
- Increase to 20mg modified-release morphine sulphate twice daily with 7.5mg immediate-release morphine sulphate on a when required, hourly basis.
At lower dose ranges (e.g. < 20mg morphine in 24 hours), an increase of 100% in the regular dose, as in the example, would be acceptable (i.e. changing from regular doses totalling 20mg/24 hours to 40mg/24 hours). Beyond these low doses, the regular dose of morphine should not be increased by more than 33–50% every 24 hours[27],[28]
. For example, it would not be considered appropriate to increase from 30mg twice daily morphine to 60mg twice daily morphine, regardless of the number of ‘as required’ morphine doses used.
Used correctly, the combination immediate-release morphine both regularly and on a when required (up to hourly) basis is an established, safe, rapid and effective means of initiation with a high average adherence rate of 85% in an oncology/palliative care population[29]
. Using this method at the described initial doses, a when required dose that matches the regular four-hourly dose is more likely to be effective and dose-related adverse effects are unlikely to be significant[30]
. It is broadly accepted to use a when required dose in the range of one-sixth to one-tenth of the total daily dose of morphine[28]
.
Yet despite its merits, a four-hourly immediate-release initiation regime is not without issues and is seen less in practice. The success of this regime relies upon:
- Subjective self-assessment;
- Manual and mental dexterity in drawing up and correctly using liquid doses (for patients or carers in their own homes);
- An expectation of expedience in those administering timely analgesia (in an inpatient setting).
It can be burdensome in a palliative demographic, where polypharmacy and frequent medicine administration times are already prevalent. Furthermore, both patient preference for unbroken sleep and timing of some inpatient medication rounds degrades four-hourly administration to a more practical four times per day (or four-hourly during waking hours as per the example above) — a pragmatic solution, but a deviation from the true four-hourly regimes the literature describes.
For these reasons, patients and prescribers may opt for the aformentioned simpler approach of starting low-dose modified-release opioids[29]
. Kaplan et al. found initiating modified-release oxycodone every 12 hours was just as effective as initiating immediate-release oxycodone four times per day in managing patients with moderate-to-severe cancer pain, while conferring fewer adverse effects[32]
. In addition, Klepstad et al. concluded in their 2003 randomised controlled trial that using sustained-release morphine was just as effective as an immediate-release, four-hourly initiation regime (although their study looked at once daily sustained-release morphine and not twice daily modified-release regimes)[33]
.
Whichever initiation method is selected, ongoing review and clinical assessment of a patient allows the prescriber to adjust and titrate the dose of opioids according to effect.
How to titrate strong opioids
For pain that remains uncontrolled despite commencement of a regular opioid, dose titration may be necessary. Titration of background doses can be guided by the total sum of breakthrough doses taken in the previous 24 hours but must not exceed an overall increase of 33–50%[28],[31]
For example, a patient taking morphine modified-release capsules 20mg twice daily is taking 40mg of morphine per day, which can be increased by a maximum of 50%. This would take the dose to 60mg of morphine per day, which could be given as morphine modified-release capsules 30mg twice daily. The patient’s when required dose should also be recalculated as one-sixth to one-tenth of the new background dose – 60mg of morphine per day divided by six equals 10mg of morphine, to be taken when required.
As a general rule, strong opioids do not have a ceiling effect and doses can continue to be titrated as long as a favourable balance between analgesia and adverse effects has been struck[34]
. Critically, titration of background doses should only be considered after careful consideration of the following:
1. Is the pain opioid-responsive?
Prescribers should be vigilant and ensure that a patient’s pain is ‘opioid-responsive’. This can be achieved by asking the individual to score their pain out of ten (with zero representing no pain and ten indicating the worst pain imaginable) and observing for an improvement following opioid administration[35]
. In patients who are unable to coherently verbalise, non-verbal signs of pain, such as grimacing, guarding, agitation and distress, can be monitored as surrogate markers for analgesic efficacy[36]
. The goals of pain management should be individualised to the patient, with behavioural and functional outcomes considered in addition to pain scores.
Titration of background doses may be appropriate for opioid-responsive pain, while re-evaluation and potential application of adjuvant analgesia (such as neuropathic agents) should be considered for pain unresponsive to opioids[31]
[37]
.
2. Is the opioid well tolerated?
The burden of adverse effects versus the analgesic efficacy of the opioid regimen should be assessed and reassessed prior to each dose titration[37],[38]
. This is because some adverse effects occur in a dose-dependent manner[38]
. For patients with opioid-responsive pain who are experiencing intolerable adverse effects (e.g. ongoing drowsiness), rotation to an alternative opioid may be warranted[39]
.
3. What type of pain is the patient experiencing?
Serum levels of opioids should ideally match the patient’s pain profile, as exposing an individual to an opioid in the absence of pain increases the likelihood of unacceptable adverse effects[37]
. This means:
- Patients experiencing constant pain, or multiple daily episodes of breakthrough pain, are best managed with regular ‘round-the-clock’ opioids;
- Short-lived predictable pain, referred to as ‘incident pain’ (e.g. pain during dressing changes) is best treated with pre-emptive analgesia[40]
. This could be achieved by administering a dose of immediate-release morphine 20–30 minutes prior to the pain-inducing activity
[40]
. Initiating or titrating ‘round-the-clock’ opioids for incident pain exposes the individual to a constant level of opioid while the pain is only present for a fraction of this time — as a result, toxicity may occur; - Acute pain (e.g. post-surgery) in patients established on regular opioids can be treated with appropriate when required doses or, if necessary, a temporary increase in the background dose. As the acute pain resolves, this should be reduced to avoid toxicity[31,]
[34],[40],[41],[42]
.
Consequently, in patients who are using several daily doses of a when required opioid, it is important to consider the type of pain they are experiencing. Pains of varying aetiology can occur concurrently, necessitating the use of multiple analgesic strategies[40]
.
For example, individuals with bone metastases can experience chronic pain and predictable movement-induced pain. In such circumstances, it is important to attribute when required usage correctly (i.e. background pain vs. incident pain), as titration of ‘round-the-clock’ doses in response to incident pain, rather than chronic pain, may lead to toxicity. The identification of incident pain may necessitate a pre-emptive dosing strategy.
4. Has steady state been achieved?
Steady state is the point at which the serum levels of a regularly administered drug stabilise[43],[44]
. It is at this point that the efficacy and tolerability of an individual’s opioid regimen can be accurately assessed[44]
. Generally, background doses should not be titrated unless sufficient time has elapsed for steady state to be achieved[31]
. Titrating prior to this risks ‘overshooting’ and precipitating unwanted adverse effects or toxicity[43],[44]
.
Prescribers must be aware that the time taken for a chosen opioid to reach steady state will vary according to the drug’s half-life, formulation (i.e. modified- or immediate-release) and the presence of renal and/or hepatic impairment[12]
[13]
[43]
- Immediate-release products include tablets, capsules or liquids which can be administered at regular intervals throughout the day (e.g. morphine or oxycodone liquid given every four hours) or an injectable product administered as a continuous subcutaneous infusion over 24 hours (e.g. a dose of morphine administered via a syringe pump)[11]
. When administered in such a fashion, steady state can be achieved after around five half-lives have elapsed (see Table 1). - Modified-release products (e.g. morphine modified-release capsules or oxycodone modified-release tablets), on the other hand, take around two to three days to achieve steady state[31]
[43]
[44]
. - Transdermal products (e.g. fentanyl and buprenorphine) take several days to achieve steady state[45],[46]
.
If, after a few adjustments in dose, an acceptable balance between side effects and pain control cannot be reached, NICE recommends that specialist advice is sought[21]
.
Opioid rotation and optimisation
The process of substituting one opioid for another in the hope of optimising therapeutic efficacy and/or reducing opioid-related adverse effects is termed opioid rotation[47]
.
The moderate-to-severe pain associated with cancer often requires increasing doses of opioids in order to achieve adequate analgesia. Over time, patients can develop tolerance to the analgesic effects of opioid medication, while remaining sensitive to the escalation in adverse effects, which are often dose-related[38]
.
The rationale underpinning opioid rotation is that of ‘incomplete cross-tolerance’ (i.e. a high level of tolerance to one opioid may not be demonstrated with an alternative one)[48]
. This cross-tolerance can apply to either desired analgesia or unwanted adverse effects, and there is a common variability – drug- and patient-related – in response[40]
. Reddy et al. studied outpatients (n=114) with cancer pain, finding that opioid rotation was successful in two-thirds of those cases, where it conferred improvements in pain and adverse effects[39]
.
Opioid rotation seeks to establish an advantageous relationship between analgesia and toxicity by identifying the most favourable opioid — assuming cross-tolerance to analgesia will be less than the cross-tolerance to drug-induced side effects[48]
. Opioid rotation may be necessary for several reasons:
- Intolerable side effects, such as hallucinations or emesis;
- Change in clinical status (e.g. new or worsening renal impairment);
- Practical issues, such as patient convenience or adherence[49],[50]
.
Higher opioid requirements in advanced cancer do not always equate to improved analgesic efficacy[49]
. Rather, dose escalation can often fail to adequately control pain and lead to worsening adverse effects[49]
. At upper dosage limits, patients are at risk of developing symptoms of opioid toxicity (e.g. sedation and hallucinations)[51]
. Rotation to a substitute opioid may be enough to continue analgesia while maintaining tolerability[49],[51]
.
Opioid excretion is affected by renal function, and the accumulation of metabolites (e.g. M6G) can result in dose-limiting toxicity[52]
. With declining renal function, rotation to an opioid with a safer renal profile can help clear metabolites and restore the balance between adverse effect and analgesic (e.g. rotating from morphine to alfentanil in an individual with renal impairment)[13]
.
Hepatic disease can alter the pharmacokinetics of opioids, particularly those such as alfentanil which undergo extensive metabolism (via CYP450) in the liver[53]
. The use of modified-release formulations in hepatic impairment may lead to worsening of adverse effects, such as sedation, as a consequence of opioid accumulation[54]
. In such circumstances, immediate-release preparations at an extended dosing interval may be preferred (e.g. immediate-release morphine given three times daily in a patient with cirrhosis)[12]
.
Rotation may be undertaken to take advantage of characteristics of another opioid, or for reasons of practicality. For example, rotation from oral morphine to transdermal fentanyl may be appropriate in somebody with short bowel syndrome who does not absorb enteral drugs effectively[55]
. Changing the dosing schedule or route of administration can also improve patient adherence[55],[56]
.
For example, a patient is taking 60mg modified-release morphine twice daily for control of pain from metastatic bowel cancer. She uses 20mg immediate-release morphine as breakthrough analgesia with excellent effect but reports suffering from hallucinations each time it is used. There are no other features of opioid toxicity, so a shared decision is made to rotate to oxycodone.
In clinical practice, oxycodone is around 1.5 times more potent than morphine (the manufacturer states it is 2 times more potent)[57]
. Therefore, 60mg modified-release morphine twice daily, with 20mg immediate-release morphine when required, would approximately convert to 40mg modified-release oxycodone twice daily, with 15mg immediate-release oxycodone when required. The patient commences oxycodone in place of morphine and their hallucinations resolve, while analgesic efficacy is maintained.
Selection, initiation and optimisation of opioids are typical points during the palliative care journey. At earlier stages in a patient’s illness, adequate analgesia can maintain function and quality of life; however, at the end of their journey, opioids for the maintenance of comfort remain just as important a focus and bring with them their own set of challenges to consider.
End-of-life management
Established opioid therapy should be continued at the end of life[22]
[31]
.Yet, continued oral administration will likely become unreliable owing to various factors, such as:
- Persistent vomiting;
- Dysphagia;
- Coma;
- Bowel obstruction;
- Poor absorption of oral drugs[22]
.
In such cases, it is appropriate to stop administering the opioid orally and change to a continuous subcutaneous infusion that can be given via a syringe pump. Conversion of regular oral opioids to a syringe pump should be done in three steps:
Step 1: Calculate the equivalent dose
At this point, the prescriber should decide if they are going to administer the same opioid via the syringe pump or if they are going to rotate to an alternative (e.g. changing from oral morphine to subcutaneous alfentanil may be prudent in an individual with declining renal function). They should then calculate the equipotent dose of the subcutaneous opioid (see Table 1
)[12],[13],[14],[15],[16],[17],[18],[19],[20]
.
For example, a patient taking morphine 30mg twice daily is taking a total of 60mg of morphine over 24 hours. Subcutaneous morphine is regarded as being twice as potent as oral morphine; therefore, the equivalent subcutaneous dose would be 30mg over 24 hours[28]
.
Step 2: Start the syringe pump at an appropriate time
The time interval between the last dose of oral modified-release opioid and the commencement of a syringe pump must be considered[31]
. If the crossover is performed too soon, serum levels may increase and precipitate adverse effects. Alternatively, if the syringe pump is delayed, pain may ensue.
When administering an opioid via a syringe pump, there is a delay before the rate of drug delivery to the serum reaches its maximum, which must be considered[58]
. If a patient has managed to take their previous dose of oral modified-release opioid, the syringe pump should be commenced two hours before the next dose would have been due[31]
. This takes into account the delay and allows the syringe pump to achieve effective serum levels from the point in time at which the next oral dose would have been taken. If a patient has missed their last dose of oral modified-release opioid, then the syringe pump can be initiated immediately[31]
.
Step 3: Calculate and prescribe an appropriate when required dose
Subcutaneous when required doses should be given at one-sixth to one-tenth of the background dose, as they are with oral doses[28],[31]
.
Additionally, subcutaneous bolus doses should not exceed a volume of 2ml, as larger volumes may be associated with pain and other adverse effects[31]
. For patients requiring large when required doses, high-strength ampoules may be used to keep the volume below 2ml.
Titrating syringe pumps
If the patient continues to experience pain, the dose of opioid in the syringe pump can be titrated as per the guidance on titrating oral strong opioids.
Summary
The term ‘palliative’ encompasses a vastly heterogenous population and whether patients are in the last 12 months or 12 hours of life, there is an increasing role for strong opioids and a diminishing relevance of weaker ones.
As the search for novel analgesic targets intensifies and evidence of wide-ranging harm from opioids is thrown unceremoniously into the spotlight, there is a need to remember opioids’ unrivalled efficacy, the vast body of guiding evidence spanning decades that supports their use, and their established and unparalleled place in the arsenal against cancer pain. To that end, this piece has provided a synthesis of practical advice to guide healthcare professionals in retaining familiarity with the nuances of opioid selection and use in the palliative demographic, so that pain and suffering can continue to be alleviated through the safe, empowered and enlightened prescribing of these medicines.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
About the authors
Jonathan Pickard is a consultant in palliative medicine at St. Benedict’s Hospice and Specialist Palliative Care Centre in Sunderland; Emma McDonald is an internal medicine trainee at Northumbria Healthcare NHS Foundation Trust; and Jonathan Hindmarsh is advanced clinical pharmacist in palliative medicine at South Tyneside and Sunderland NHS Foundation Trust.
References
[1] Stein C. New concepts in opioid analgesia. Expert Opin Investig Drugs 2018;27(10):765–775. doi: 10.1080/13543784.2018.1516204
[2] Els C, Jackson TD, Kunyk D et al. Adverse events associated with mediumâ€and longâ€term use of opioids for chronic nonâ€cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2017;10(10):CD012509. doi: 10.1002/14651858.CD012509
[3] Manchikanti L, Kaye AM, Knezevic NN et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician 2017;20(2):S3–92. PMID: 28226332
[4] World Health Organization. WHO’s analgesic ladder (reproduced for The Pharmaceutical Journal). 2019. Available at: https://www.pharmaceutical-journal.com/acute-pain/clinical-guidelines-and-evidence-base-for-acute-pain-management/20206653.article?firstPass=false (accessed November 2020)
[5] General Medical Council. Treatment and care towards the end of life: good practice in decision making. 2010. Available at: https://www.gmc-uk.org/-/media/documents/treatment-and-care-towards-the-end-of-life—english-1015_pdf-48902105.pdf?la=en&hash=41EF651C76FDBEC141FB674C08261661BDEFD004 (accessed November 2020)
[6] Fallon M, Giusti R, Aielli F et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018;29(Suppl4):iv166–iv191. doi: 10.1093/annonc/mdy152
[7] Bandieri E, Romero M, Ripamonti CI et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol 2016;34(5):436–442. doi: 10.1200/JCO.2015.61.0733
[8] Principles of use of analgesics. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[9] Codeine phosphate. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[10] Findlay JW, Jones EC, Butz RF & Welch RM. Plasma codeine and morphine concentrations after therapeutic oral doses of codeineâ€containing analgesics. Clin Pharmacol Ther 1978;24(1):60–68. doi: 10.1002/cpt197824160
[11] Strong Opioids. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[12] Hepatic impairment. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[13] Ashley C & Dunleavy A. The renal drug handbook: the ultimate prescribing guide for renal practitioners. Florida: CRC Press; 2017
[14] McQuay HJ, Carroll D, Faura CC et al. Oral morphine in cancer pain: influences on morphine and metabolite concentration. Clin Pharmacol Ther 1990;48(3):236–244. doi: 10.1038/clpt.1990.145
[15] Klimas R & Mikus G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: a quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br J Anaesth 2014;113(6):935–944. doi: 10.1093/bja/aeu186
[16] Scholz J, Steinfath M & Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin Pharmacokinet 1996;31(4):275–292. doi: 10.2165/00003088-199631040-00004
[17] North-Lewis P. Drugs and the Liver: A Guide to Drug Handling in Liver Dysfunction. London: Pharmaceutical Press; 2008
[18] Hasselstrom J, Eriksson S, Persson A et al. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol 1990;29(3):289–297. doi: 10.1111/j.1365-2125.1990.tb03638.x
[19] Tallgren M, Olkkola KT, Seppälä T et al. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther 1997;61(6):655–661. doi: 10.1016/S0009-9236(97)90100-4
[20] Löfdal KC, Andersson ML & Gustafsson LL. Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs 2013;73(6):533–543. doi:10.1007/s40265-013-0036-0
[21] National Institute for Health and Care Excellence. Palliative care for adults: strong opioids for pain relief. Clinical guideline [CG140]. Available at: https://www.nice.org.uk/guidance/cg140 (accessed November 2020)
[22] Continuous subcutaneous drug infusions. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary , 6th ed. London: Pharmaceutical Press; 2017
[23] Broadbent AM, Heaney A & Weyman K. A review of short bowel syndrome and palliation: a case report and medication guideline. J Palliat Med 2006;9(6):1481–1491. doi.org/10.1089/jpm.2006.9.1481
[24] Preston CL. Stockley’s drug interactions. London: Pharmaceutical Press; 2015
[25] Liukas A, Hagelberg NM, Kuusniemi K et al . Inhibition of cytochrome P450 3A by clarithromycin uniformly affects the pharmacokinetics and pharmacodynamics of oxycodone in young and elderly volunteers. J Clin Psychopharmacol 2011;31(3):302–308. doi:10.1097/JCP.0b013e3182189892
[26] Tramadol. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[27] Joint Formulary Committee. British National Formulary, 79th ed. London: BMJ Group and Pharmaceutical Press; 2020
[28] Morphine. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[29] De Conno F, Ripamonti C, Fagnoni E et al. The MERITO Study: a multicentre trial of the analgesic effect and tolerability of normal-release oral morphine during ‘titration phase’ in patients with cancer pain. Palliat Med 2008;22(3):214–221. doi: 10.1177/0269216308088692
[30] Hanks GW, De Conno F, Ripamonti C et al. Morphine in cancer pain: modes of administration. BMJ 1996;312(7034):823–826. doi: 10.1136/bmj.312.7034.823
[31] NHS. Northern England Clinical Networks. Palliative and End of Life Care Guidelines. 2016. Available at: http://www.northerncanceralliance.nhs.uk/wp-content/uploads/2018/11/NECNXPALLIATIVEXCAREX2016-1.pdf (accessed November 2020)
[32] Kaplan R, Parris WC, Citron ML et al. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. J Clin Oncol 1998;16(10):3230–3237. doi: 10.1200/JCO.1998.16.10.3230
[33] Klepstad P, Kaasa S, Jystad Ã… et al. Immediate-or sustained-release morphine for dose finding during start of morphine to cancer patients: a randomized, double-blind trial. Pain 2003;101(1–2):193–198. doi: 10.1016/S0304-3959(02)00328-7
[34] Krakowski I, Theobald S, Balp L et al. Summary version of the standards, options and recommendations for the use of analgesia for the treatment of nociceptive pain in adults with cancer (update 2002). Br J Cancer 2003;89(Suppl1):S67–72. doi:10.1038/sj.bjc.6601086
[35] Breivik H, Borchgrevink PC, Allen SM et al. Assessment of pain. Br J Anaesth 2008;101(1):17–24. doi: 10.1093/bja/aen103
[36] Abbey J, Piller N, Bellis AD et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004;10(1):6–13. doi: 10.12968/ijpn.2004.10.1.12013
[37] Mercadante S. Opioid titration in cancer pain: a critical review. Eur J Pain 2007;11(8):823–830. doi: 10.1016/j.ejpain.2007.01.003
[38] Rogers E, Mehta S, Shengelia R & Reid MC. Four strategies for managing opioid-induced side effects in older adults. Clin Geriatr 2013;21(4). PMID: 25949094
[39] Reddy A, Yennurajalingam S, Pulivarthi K et al. Frequency, outcome, and predictors of success within 6 weeks of an opioid rotation among outpatients with cancer receiving strong opioids. Oncologist 2013;18(2):212–220. doi: 10.1634/theoncologist.2012-0269
[40] Mercadante S & Portenoy RK. Opioid poorly-responsive cancer pain. Part 1: clinical considerations. J Pain Symptom Manage 2001;21(2):144–150. doi: 10.1016/s0885-3924(00)00228-1
[41] Mehta V & Langford RM. Acute pain management for opioid dependent patients. Anaesthesia 2006;61(3):269–276. doi: 10.1111/j.1365-2044.2005.04503.x
[42] Management of postoperative pain in opioid-dependent patients. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[43] Mandema JW, Kaiko RF, Oshlack B et al. Characterization and validation of a pharmacokinetic model for controlledâ€release oxycodone. Br J Clin Pharmacol 1996;42(6):747–756. doi: 10.1046/j.1365-2125.1996.00481.x
[44] Hacker M, Messer WS & Bachmann KA. Pharmacology: principles and practice. Cambridge: Academic Press; 2009
[45] Grond S, Radbruch L & Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet 2000;38(1):59–89. doi: 10.2165/00003088-200038010-00004
[46] Transdermal patches. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[47] Fine PG & Portenoy RK. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage 2009;38(3):418–425. doi: 10.1016/j.jpainsymman.2009.06.002
[48] Fallon M. Opioid rotation: does it have a role? Palliat Med 1997;11(3):177–178. doi: 10.1177/026921639701100301
[49] Smith HS & Peppin JF. Toward a systematic approach to opioid rotation. J Pain Res 2014;7:589–608. doi: 10.2147/JPR.S55782
[50] Mercadante S. Predictive factors and opioid responsiveness in cancer pain. Eur J Cancer 1998;34(5):627–631. doi: 10.1016/s0959-8049(97)10053-3
[51] de Stoutz ND, Bruera E & Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage 1995;10(5):378–384. doi: 10.1016/0885-3924(95)90924-c
[52] Sande TA, Laird BJ & Fallon MT. The use of opioids in cancer patients with renal impairment—a systematic review. Support Care Cancer 2017;25(2):661–675. doi: 10.1007/s00520-016-3447-0
[53] Tegeder I, Lötsch J & Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet 1999;37(1):17–40. doi: 10.2165/00003088-199937010-00002
[54] Soleimanpour H, Safari S, Nia KS et al. Opioid drugs in patients with liver disease: a systematic review. Hepat Month 2016;16(4):e32636. doi: 10.5812/hepatmon.32636
[55] McCarberg BH & Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther 2001;8(3):181–186. doi:10.1097/00045391-200105000-00006
[56] Timmerman L, Stronks DL, Groeneweg JG & Huygen FJ. Prevalence and determinants of medication nonâ€adherence in chronic pain patients: a systematic review. Acta Anaesthesiol Scand 2016;60(4):416–431. doi: 10.1111/aas.12697
[57] Oxycodone. In: Twycross G, Wilcock A, Howard P (eds.). Palliative Care Formulary, 6th ed. London: Pharmaceutical Press; 2017
[58] Stuartâ€Harris R, Joel SP, McDonald P et al. The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine. Br J Clin Pharmacol 2000;49(3):207–214. doi: 10.1046/j.1365-2125.2000.00141.x