ISM / Science Photo Library
Inflammatory bowel (IBD) disease refers to two distinct conditions: Crohn’s disease (CD) and ulcerative colitis (UC). Both are chronic relapsing and remitting conditions that can result in considerable long-term morbidity.
IBD is typically a disease of young people. The peak age of diagnosis of 10–25 years, with a second peak at 50 years. Current estimates of the number of people with IBD in the UK vary significantly. In 2012, the National Institute for Health and Care Excellence (NICE) estimated around 260,000 people had CD[1]
or UC[2]
, whereas in the same year the National IBD Standards Group estimated around 620,000 people had IBD, costing the NHS as much as £470m per year[3]
.
The precise mechanisms underlying the development of IBD are not known, although it is believed to be a complex interplay of genetic, environmental and immunological factors; people who have a first-degree relative with IBD are ten times more likely to develop the condition[4]
.
Potential external factors have been researched extensively, including smoking, stress, antibiotic use and vitamin D levels[5]
. The results have largely been inconclusive, although smoking appears to be associated with a two-fold increase in risk and severity of CD but seems to have a protective effect in UC. Air pollution has also been highlighted as a possible cause of the increase in IBD between 1940 and 1980 in US population-based studies, although this link is not proven.
The effects of diet on established IBD remains poorly understood.
Clinical features
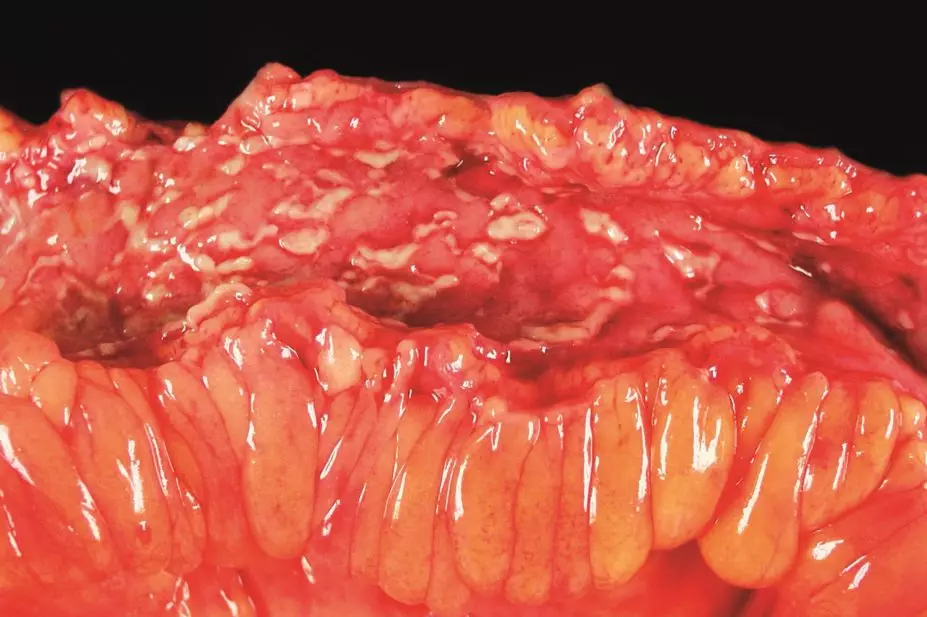
UC affects the colon and rectum, and causes inflammation and tissue damage to the mucosal and, to a lesser degree, the submucosal layers of the intestinal lining. A flare is characterised by excessive watery stools and bloody diarrhoea, with continuous inflammation and ulceration of the mucosa, starting at the distal end of the colon.
CD can affect the entire intestinal tract, from the mouth to the anus, and ulceration is transmural (i.e. can affect all layers of the intestinal wall). Deep ulcers, called skip lesions, occur within healthy tissue throughout the whole intestine and can be seen on endoscopy. Symptoms of an acute flare include abdominal pain, weight loss and diarrhoea (which can contain some blood and mucus).
Long-term complications of CD include the development of strictures, fistulas and perianal disease. Strictures, narrowed segments of the bowel, are common and can lead to blockages, acute dilatation and perforation. Fistulas are abnormal channels lined with granulation tissue that can form between the intestine and the skin, organs such as the bladder or vagina, or other parts of the intestine.
Patients with IBD have an increased risk of colon cancer, particularly if they do not adhere to treatment.
Optimising therapy
Medicines used to treat UC and CD include corticosteroids, aminosalicylates, immunomodulators (such as azathioprine, mercaptopurine, methotrexate and ciclosporin) and biologics.
There are many ways IBD treatments can be optimised. These can include:
- Using an appropriate dose and reduction regimen of corticosteroids during acute flares, and co-prescribing calcium and vitamin D to prevent osteoporosis and other adverse effects
- Advising patients taking aminosalicylates how to adjust their dose when experiencing a flare, and how to reduce their dose to maintenance therapy during remission
- Selecting the appropriate formulation of corticosteroid or aminosalicylate for the site of disease (e.g. suppositories for the rectum, foam for the sigmoid colon, enema for the descending colon)
- Supporting adherence to therapy; recent studies have shown non-adherence to be one of the main factors contributing to relapse[5]
- Planning how to stop medicines for patients who are in clinical remission
- Developing ‘rescue strategies’ for patients who have stopped treatment and relapsed
- Using therapeutic drug monitoring to optimise treatment with thiopurines and biologics.
Corticosteroids
Flares are usually treated with corticosteroids. Current guidelines recommend a course of prednisolone 40mg daily, reduced by 5mg weekly. Initial doses of predisolone 15mg or less, faster reduction regimens or short pulses of corticosteroids are ineffective in controlling a flare.
Around 10–20% of patients develop corticosteroid dependency. These patients experience flares as soon as the corticosteroid dose is reduced below a particular threshold or stopped entirely.
Rectal preparations are less acceptable to many patients but are an effective treatment option, especially in distal disease. Rectal foam preparations generally do not spread as far in the colon as enemas, but are more acceptable to patients. Enemas tend to bypass the rectum and are not as effective as suppositories in patients with proctitis.
Patients with acute severe disease require admission to hospital, where they receive treatment with intravenous hydrocortisone 100mg four times a day. Treatment is stepped up, and a biologic (or ciclosporin in UC) is often prescribed. Surgical interventions are considered if there is no marked improvement after three days.
Mesalazine
Mesalazine is used to treat mild to moderate UC flares at doses of ≥4g per day, and for the maintenance of remission in UC at doses of ≥2g per day. In addition to its therapeutic benefits, taking mesalazine for UC reduces the patient’s risk of developing colorectal cancer by 75%.
Current guidelines advise a mesalazine liquid enema (1g per day) should be used as adjunct therapy when treating acute flares[6]
. There is little evidence that mesalazine is effective in CD, but there is some evidence that high doses (≥ 4g/day) can be used to prevent flares following surgery for CD[7]
.
Taking mesalazine once daily for UC is as effective as taking multiple daily doses, and single doses should be used to improve patient adherence. The BNF states that there is little difference in terms of efficacy between mesalazine preparations, and the choice should be based on patient preference. There is some evidence of differences in mucosal healing (as shown on endoscopy) between the preparations available, but further study is required to determine whether this is of clinical significance.
There is a 3.5-fold increase in the number of flares experienced by patients who switch preparations[5]
.
Thiopurines
Thiopurines, such as azathioprine and mercaptopurine, remain the first-line immunomodulators used for the treatment of IBD as recommended by the British Society of Gastroenterology.
Azathioprine is a prodrug that is metabolised to mercaptopurine, which in turn is metabolised by a series of enzymes into pharmacologically active thioguanine nucleotides (TGN). Around 20% of patients who do not tolerate azathioprine can tolerate mercaptopurine.
Mercaptopurine is also metabolised by the enzyme thiopurine methyltransferase (TPMT), which produces methylmercaptopurine (MeMP), and xanthine oxidase, which produces thiouric acid. Neither metabolite is pharmacologically active.
Patients who have high levels of TPMT can produce high levels of MeMP, which can cause hepatotoxic side effects. High levels of TGN can cause dose-related adverse effects, such as myelosuppression.
TPMT levels should therefore be measured before a patient starts treatment with a thiopurine (see ‘TPMT levels’).
TPMT levels
TPMT levels should be measured before a patient starts a thiopurine, and the starting dose should be adjusted. TPMT is only measured at the start of treatment to inform the initial dose and identify patients at increased risk of adverse effects, such as myelosuppression or liver dysfunction. TPMT measurements are not used in dose optimisation.
Thiopurine methyltransferase levels
- <10mU/L Deficient
- 20–67mU/L Low
- 68–150mU/L Normal
- >150mU/L High
A starting dose of 2–2.5mg/kg/day of azathioprine or 1–1.5mg/kg/day of mercaptopurine is recommended for patients with normal or high TPMT levels.
Patients with reduced TPMT levels should be started on half the recommended initiation doses as they are at greater risk of myelosuppression. Thiopurines should be avoided in TPMT deficient patients.
Recent studies[8]
have shown that therapeutic drug monitoring of the metabolites TGN and MeMP allows for patients who were previously considered intolerant of or not responding to thiopurines to remain on treatment.
Pharmacist-led drug monitoring clinics measure TGN and MeMP levels four weeks after treatment with thiopurines is started to optimise outcomes. Patients should also have their TGN and MeMP levels measured if they are not responding to treatment.
The recommended range is 200–400 pmol/8×108 red blood cells for TGN, and <5,700 pmol/8×108 red blood cells for MeMP.
If levels of both metabolites are low or absent, it is likely that the patient is not adhering to therapy and the patient should be asked if they are taking the medicine as directed.
If levels of both metabolites are low and the patient is taking the medicine, the patient is being undertreated and the dose of thiopurine should be increased in steps, guided by the patient’s TGN results. Levels should be checked again four weeks after any dose change, which ensures the levels are measured at steady state.
If TGN levels are low and MeMP levels are high, the patient may not respond to therapy and is at risk of hepatoxicity. Allopurinol can be co-prescribed in these patients, as this inhibits xanthine oxidase and possibly the methylating pathways, resulting in a higher ratio of thiopurine converted to TGN. If allopurinol is co-prescribed, the dose of the thiopurine should be reduced by 25% to avoid excessive TGN levels.
Patients with high levels of both TGN and MeMP who are not responding to treatment should stop taking thiopurines and alternative therapies should be considered.
The patient should also have full blood counts and liver function tests (LFTs) every two weeks for the first three months of treatment, then three-monthly thereafter. Low white blood cell counts or abnormal LFTs may be caused by high TGN or MeMP levels respectively. These levels can often be normalised without stopping treatment by optimising therapy.
Biologics
Biologic medicines for the treatment of IBD include infliximab, adalimumab and golimumab. Certolizumab was given a negative opinion by the European Medicines Agency Committee for Medicinal Products for Human Use for IBD. Vedolizumab, an anti-integrine, and biosimilars of infliximab are expected to be licensed in 2015.
A recent study found that up to 40% of patients with CD do not respond to treatment with biologics, 30% to 50% achieve complete remission after six months and 30% of patients maintain the response for 12 months with continual treatment[7]
. Current strategies to overcome loss of response involve increasing the dose, decreasing the interval between administrations or switching to an alternative agent.
An alternative strategy is to optimise the use of biologics using therapeutic drug monitoring. The therapeutic drug monitoring of infliximab (TAXIT) trial[9]
investigated the value of optimising infliximab treatment through therapeutic drug monitoring and measuring the level of antibodies to infliximab (ATI) for patients who were in clinical remission. The target trough level of infliximab (TLI) is between 3µg/ml and 7µg/ml. Baseline characteristics of the study population during the optimisation phase of the trial found 44% of patients were within the target range, 26% had levels >7µg/ml, 21% had levels <3µg/ml and 9% of the cohort had undetectable levels (75% of whom tested ATI positive).
In the initial phase of the trial, all patients had their treatment optimised to achieve TLIs in the target range. During this optimisation phase, dose escalation to target TLI for suboptimal patients resulted in better initial disease control, and dose de-escalation for patients with high TLI did not cause a change in disease control but reduced exposure to infliximab.
Results from the maintenance phase of the TAXIT trial — in which patients were randomised to continue TLI-based dosing or receive standard care (clinical-based dosing) — did not show superior remission rates but allowed infliximab to be used more efficiently.
The STORI trial[10]
identified a group of patients treated with infliximab and a thiopurine that may stop treatment with a low risk of relapse within one year. Patients in the trial stopped treatment with the medicines after six months of remission (both clinical remission and on investigation with endoscopy). The average relapse rate was 58%, but patients with two or fewer identified risk factors had a relapse risk of 14–16% only (see ‘STORI predictive factors for relapse’). The study also found that restarting treatment with infliximab if symptoms relapse is successful in 98% of patients if done within six months of stopping treatment.
STORI predictive factors for relapse
- Male sex
- No previous surgical resection
- White blood cell count > 6×109/L
- Haemoglobin ≤ 145g/L
- C-reactive protein ≥ 5.0mg/L
- Fecal calprotectin ≥ 300μg/g
Two or fewer risk factors: relapse risk 14–16%
NICE is currently developing guidance on the use of therapeutic drug monitoring for biologics. Stratification of patients for optimal response to biologics is still in its infancy and large patient registry studies over several years are required to determine which disease symptoms, patient characteristics or genetic markers influence response and outcome.
Anja St.Clair Jones is lead pharmacist for surgery and digestive services at Brighton and Sussex University Hospitals NHS Trust.
References
[1] National Institute for Health and Clinical Excellence. Crohn’s disease: Management in adults, children and young people. London: NICE 2012.
[2] National Institute for Health and Clinical Excellence. Ulcerative colitis: Management in adults, children and young people. London: NICE 2012.
[3] IBD Standards Group UK. Standards for the healthcare of people who have inflammatory bowel disease. 2013 update. London: Oyster Healthcare Communications 2013.
[4] Russell RK & Satsangi J. Does inflammatory bowel disease run in families? Inflamm Bowel Dis 2008;14(S2):20–21.
[5] Robinson A, Hankins M, Wiseman G et al. Maintaining stable symptom control in inflammatory bowel disease: a retrospective analysis of adherence, medication switches and risk of relapse. Aliment Pharmacol Ther 2013;38:531–538.
[6] St Clair Jones A. Mesalazine in the treatment of ulcerative colitis. HPE 2012;63:33–37.
[7] Mowat C, Cole A, Windsor A et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011. doi:10.1136/gut.2010.224154.
[8] Smith M, Blaker P, Marinaki AM et al. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohn’s and Colitis 2012;6(9):905–12.
[9] Vande Casteele N, Compernolle G, Ballet V et al. Individualised infliximab treatment using therapeutic drug monitoring: A prospective controlled trough level adapted infliximab treatment (TAXIT) trial. European Crohn’s and Colitis Organisation oral presentation 2012.
[10] Louis E, Mary JY, Vernier-Massouille G et al. Maintenance of Remission Among Patients With Crohn’s Disease on Antimetabolite Therapy After Infliximab Therapy Is Stopped. Gastroenterology 2012;142:63–70.