This content was published in 2010. We do not recommend that you make any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Immunosuppressant medicines have allowed patients with end-stage organ failure to benefit from organ transplantation (ie, experience reduced rates of morbidity and mortality, and improved quality of life). The experience gained from prescribing immunosuppressants for patients receiving a kidney transplant has contributed to treatment strategies in other forms of transplant and thus improved graft survival rates. However, this benefit must be balanced against the short- and long-term adverse effects of these medicines (eg, cardiac disease, infections and cancer) that can reduce patient survival. In this article, immunosuppression in the context of renal transplantation is explored. Although this knowledge can be extrapolated to other types of transplant, treatment protocols vary depending on the organ transplanted. Organ rejection is defined as an immune response that mediates injury to and destruction of transplanted tissue. Box 1 illustrates the mechanism by which rejection occurs.
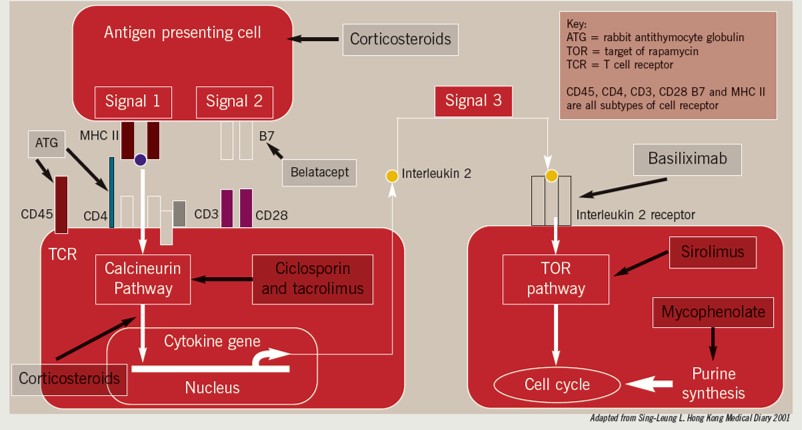
Immunosuppression regimens aim to prevent and treat organ rejection. Immunosuppressant regimens can be subdivided into those given:
- At induction (ie, at the time of transplantation)
- For maintenance
- For the treatment of rejection
All act on different aspects of the rejection pathway as shown in Box 1. Although most graft rejection is mediated by T lymphocytes, B lymphocytes can also be involved. Consequently, medicines that suppress B lymphocyte activity, such as rituximab, are being used more frequently to treat antibody-mediated rejection.
Induction immunosuppression
In the first few weeks following a transplant, acute rejection and delayed graft function can occur. Either of these can reduce the duration of graft survival or cause graft loss. Induction immunosuppression can reduce the risks of such adverse events and, therefore, is given at the time of surgery to most patients who undergo renal transplantation. (This treatment might not be needed in cases where the donor and recipient are a perfect match —ie, when they are twins.) There are two medicines licensed for induction immunosuppression in the UK — basiliximab and rabbit antithymocyte immunoglobulin (commonly called antithymocyte globulin [ATG]). Alemtuzumab is another option but its use in this context is off-label.
Basiliximab
Basiliximab is a monoclonal antibody that binds specifically to and inhibits the alpha chain of the interleukin 2 receptor (also known as the CD25 antigen)on the surface of T lymphocytes [1]. It has proved to be effective in reducing acute rejection rates compared with placebo and, aside from its risk (albeit rare) of causing hypersensitivity reactions, it has a favourable safety profile [2]. According to the National Institute for Health and Clinical Excellence, basiliximab can be used whenever it is deemed to be beneficial for the patient.
Rabbit antithymocyte immunoglobulin
The polyclonal antibody ATG has been used for many years for the treatment of organ rejection. In 2008, it was licensed in the UK as an induction immunosuppressant for patients who have undergone a renal transplant. Its mechanism of action is to cause T lymphocyte depletion by apoptosis, complement-dependent lysis and antibody-dependent cytotoxicity. One potential advantage of ATG over basiliximab is that the potency of its effect allows the introduction of maintenance immunosuppression —usually with calcineurin inhibitors (CNIs) — to be delayed. This is beneficial since CNIs can increase the risk of delayed graft function if used during the early post-transplant period [3].
Due to the high potency of ATG, leucopenia is a common side effect which, to resolve, often requires dose reduction or omission. Other adverse effects include anaphylaxis, fever, chills and respiratory distress, some of which can be ameliorated with paracetamol, hydrocortisone and chlorphenamine. ATG can also increase the risk of malignancies specifically post-transplant lymphoproliferative disease, although it is still unclear whether this is dose-related (higher doses are used for the treatment of rejection compared with prophylaxis).
NICE is yet to evaluate the use of ATG for induction therapy in renal transplantation. The Scottish Medicines Consortium is not recommending its use to prevent graft rejection for patients undergoing renal transplantation. It states that although ATG is associated with lower acute rejection rates compared with interleukin 2-receptor inhibitors, this has yet to translate into improved patient or graft survival (measured 12 months after transplant). Consequently, many transplant centres have been slow to adopt its use. Nonetheless, some renal centres are prescribing it, specifically for patients receiving organs from non-heart-beating donors. For these patients, a delay to the introduction of CNI treatment is particularly beneficial.
Alemtuzumab
The humanised monoclonal antibody alemtuzumab targets the CD25 cell surface receptor expressed on most T and B lymphocytes. It causes profound, rapid and effective depletion of peripheral and central lymphoid cells.4Its high potency might allow for a lower dose of CNI or corticosteroid to be used as maintenance treatment (known as “CNI minimisation” or “steroid sparing” regimens, respectively) [5].
Similarly to ATG, infusion-related reactions, such as anaphylaxis, fever, chills and respiratory distress, are common. Leucopenia and thrombocytopenia can also occur. Studies have shown acute rejection rates during the first year post-transplant to be lower with alemtuzumab than with basiliximab; however, long-term patient and graft survival data to support the use of alemtuzumab are lacking.
Although studies have shown all three induction treatments to be more effective than placebo at reducing rates of acute rejection, no definitive trials have shown which one produces the best long-term outcomes. At present, the most common induction treatment used in the UK is basiliximab. However, the use of ATG and alemtuzumab is expected to rise in the future as the number of organs from non-heart-beating donors increases.
Maintenance immunosuppression
For most patients, the backbone of immunosuppressive therapy is CNIs, of which there are currently two licensed in the UK: ciclosporin and tacrolimus. Since there is no conclusive evidence as to which CNI is the most effective at preventing graft rejection, NICE recommends that clinicians should select a CNI after discussing the relative side-effect profiles of each medicine with the patient.
Calcineurin inhibitors
Calcineurin normally enhances phosphatase activity in the nucleus of activated T cells, and thereby increases T cell proliferation. It also migrates to the nucleus to initiate the transcription of interleukin 2.
Ciclosporin and tacrolimus combine with binding proteins (cyclophilin and FK506 binding protein, respectively) to inhibit calcineurin activity, thus preventing proliferation of T cells [4].
CNIs are associated with several side effects (see Box 2) and will eventually (often after more than 10 years) cause chronic allograft nephropathy (ie, rejection of the transplanted kidney due to nephrotoxicity) in all transplant patients.
Box 2: Side effects of calcineurin inhibitors
CICLOSPORIN
- Hirsutism
- Hypertrichosis
- Gingival hypertrophy
- Hypertension
- Hyperkalaemia
- Nausea and vomiting
TACROLIMUS
- Glucose intolerance
- Neurotoxicity
- Alopecia
- Hyperlipidaemia
- Tremors
The main reasons for switching from ciclosporin to tacrolimus are toxicity (unacceptable side effects despite normal therapeutic blood levels), cosmetic side effects or graft rejection. The main reason for switching to ciclosporin is that tacrolimus is associated with an increased risk of developing post-transplant diabetes, known as “new onset diabetes after transplant”(NODAT). Patients who are unable to tolerate either CNI are switched to sirolimus (see below).
Therapeutic drug monitoring
For each CNI, trough blood levels are taken regularly and the dosage titrated accordingly. Ciclosporin levels during the first three months post-transplant need to be 150–200mg/ml; for tacrolimus, the desired level is 8–12μg/ml (although local guidelines vary). After three months, the desired levels are often reduced by about 25%. In cases where a patient has proven CNI toxicity, lower levels are maintained.
Several medicines can affect the blood levels of both CNIs — specifically inducers or inhibitors of cytochrome P450 3A4 (see Box 3).
Box 3: Calcineurin inhibitor interactions
INCREASE LEVELS
- Fluconazole
- Erythromycin
- Clarithromycin
- Verapamil
- Diltiazem
DECREASE LEVELS
- Rifampicin
- Phenytoin
- Carbamazepine
- St John’s wort
- Warfarin
Other medicines can increase the risk of nephrotoxicity (eg, non-steroidal anti-inflammatory drugs) or potentiate adverse effects (eg, simvastatin increases the risk of rhabdomyolysis) and should therefore be avoided. The specialist transplant team should be informed of any medicines that are prescribed concurrently for all post-transplant patients.
Brand specificity
Generic ciclosporin is now available in the UK. However, pharmacists should not change the brand of ciclosporin dispensed to patients because differences in bioavailability can compromise graft function.
Two brands of tacrolimus are also available: a twice-daily formulation (Prograf) and a once-daily formulation (Advagraf). Serious medication errors have occurred as a result of patients being dispensed the wrong brand, and substitution should only take place under close supervision of a transplant specialist.
CNI minimisation
Adjunctive immunosuppressant medicines (eg, mycophenolate, azathioprine and corticosteroids) are used in combination with CNIs in most cases. However, triple-therapy immunosuppression (ie, mycophenolate or azathioprine with a CNI and a corticosteroid) is associated with increased rates of diabetes, heart disease, dyslipidemia, bone disease and malignancy. As a result, a greater emphasis is being placed on investigating CNI minimisation (eg, by using sirolimus, see below) and early corticosteroid withdrawal strategies to improve patient survival by reducing long-term adverse effects.
Sirolimus
Sirolimus inhibits T cell activation by blocking intracellular signal transduction. Studies suggest that sirolimus binds to the specific cytosolic protein FK binding protein (FKBP)-12. The resulting FKBP-12-sirolimus complex inhibits the activation of the mammalian target of rapamycin (mTOR), a kinase critical in the cell life cycle.
Sirolimus is currently the only mTOR inhibitor licensed for maintenance immunosuppression (everolimus is licensed for treating renal cell carcinoma). It is usually reserved for use until at least three months after a transplant operation because it can delay wound healing and can cause the formation of lymphoceles (cystic masses containing lymph from damaged lymphatic channels).
Side effects
Sirolimus is not associated with long-term nephrotoxicity, hence interest has grown regarding its use in CNI minimisation or switching protocols. A recent study suggested that patients who used low-dose sirolimus regimens immediately after transplant experienced higher rates of acute rejection than did those who used low-dosetacrolimus [6]. Therefore, its use is unlikely to expand beyond the CNI switching scenario. Such switches should only be done for patients with good graft function and no proteinuria.
Adverse effects attributed to sirolimus include hyperlipidaemia, hypertriglyceridaemia, leucopenia, anaemia and joint pain. Therapeutic drug monitoring is also essential and doses are adjusted accordingly, similarly to CNIs, to a target concentration of 8–10ng/ml. Medicines that are metabolised via CYP3A4, or are inhibitors or inducers of this enzyme, can interact with sirolimus and should be used with caution.
Mycophenolate mofetil
Mycophenolate mofetil is an ester prodrug of mycophenolic acid (MPA), which inhibits the enzyme inosine monophosphate dehydrogenase (required for guanosine synthesis) and thus impairs T and B cell proliferation [4]. Mycophenolate has largely replaced azathioprine since it is associated with better graft outcomes in clinical studies (although not all patients can tolerate the dose used in this research).
The recommended dose of mycophenolate is 1g twice daily although higher doses (eg, 1.5g twice daily) are usually required for patients of Afro-Caribbean origin due to the increased rate at which they metabolise MPA.
Blood levels of MPA can be affected by concomitant administration of other immunosuppressants. Ciclosporin can lower levels through enterohepatic recirculation, whereas sirolimus (and to a lesser extent tacrolimus)increases MPA levels — thus requiring the dose of mycophenolate to be reduced [5]. MPA levels can be measured but most transplant consultants judge levels using patients’ white cell counts and gastrointestinal side effects.
Adverse effects with mycophenolate include nausea, vomiting and diarrhoea, all of which can be reduced in severity by splitting the dose during the day or, if possible, through dose reduction. Leucopenia is another common adverse effect, which can be resolved by dose reduction. Where dose reduction does not alleviate adverse effects, patients are often switched to azathioprine to ensure graft survival is not compromised. An enteric-coated version of mycophenolate is also available that, in some patients, has an improved gastrointestinal side effect profile.
Azathioprine
Azathioprine was one of the first immunosuppressants used in combination with corticosteroids for patients who had received a kidney transplant. It is a derivative of 6-mercaptopurine and functions as an antimetabolite to decrease DNA and RNA synthesis and reduce the production of lymphocytes.4Side effects include leucopenia, thrombocytopenia, cholestasis and alopecia. Azathioprine’s rate of metabolism is reduced significantlyby allopurinol — which is often prescribed for patientswho are on dialysis. Azathioprine should not be used inconjunction with allopurinol except under the supervisionof a transplant specialist.
Corticosteroids
Methylprednisolone is given at induction to all patients undergoing an organ transplant; prednisolone is used for maintenance and organ rejection. Corticosteroids work by preventing the production of interleukins 1 and 6 by macrophages and inhibit all stages of T cell activation [4]. There are many side effects associated with long-term corticosteroid use, such as Cushing’s syndrome, cataracts, bone disease, diabetes and hyperlipidaemia. This has prompted many transplant centres to use steroid withdrawal regimens but this must be carefully balanced against the potential risk of acute rejection. Research suggests that the earlier corticosteroid treatment can be withdrawn (ideally within the first few weeks), the better.
Rejection
Approximately 20% of all transplant patients will suffer an episode of rejection in their transplant life, of which 90% can be treated successfully. Rejection is diagnosed primarily on biopsy and rising creatinine and can take on various forms and severity. Treatment is based on the type and severity of rejection (see Banff criteria, Box 4).
Box 4: Banff criteria for graft rejection [7]
A simplified version of Banff diagnostic criteria — used to determine the severity of renal transplant rejection — is outlined below. The classification refers to changes identified from renal allograft biopsies. Class 1 is the least severe; class 4 is the most severe; classes 5 and 6 are not linked to severity.

Treatment of rejection
For milder cases of rejection (usually class 2 to 3 according to Banff criteria), pulse doses of methylprednisolone have been shown to be effective. For patients with more severe rejection (usually class 3 to 4 according to Banff criteria), or who are resistant to corticosteroid treatment, ATG is the preferred option. Treatment is given over a 10-day period, but since ATG can cause profound lymphopenia doses might need to be omitted. (Mycophenolate can also cause leucopenia so should be omitted during a course of treatment with ATG.) Rejection that continues to occur despite the above treatment might benefit from repeated courses or changes in maintenance immunosuppression.
Antibody-mediated rejection
Patients who have developed HLA antibodies (anti-HLA) to the transplanted organ are at greater risk of developing antibody-mediated rejection — the most severe form of rejection. In particular, those who have had previous transplants, blood transfusion or multiple pregnancies. Immunoglobulins and rituximab are available to treat such rejection — usually prescribed in conjunction with plasmapheresis (a technique that removes antibodies from a patient’s bloodstream).
Intravenous immunoglobulin has several effects on the immune system that, essentially, inhibit anti-HLA production and produce long-term suppression or elimination of anti-HLA-reactive T and B cells.4Rituximab is a monoclonal antibody directed againstCD20 antigen on B lymphocytes. The rapid and sustained depletion of circulating and tissue-based B cells that occurs following its administration has shown to be effective in treating antibody-mediated rejection [4].
Chronic interstitial fibrosis and tubular atrophy
Unless there are underlying reasons for declining graft function — such as treatment non-adherence or “acute-on-chronic rejection” — the treatment of chronic interstitial fibrosis and tubular atrophy (class V according to Banff criteria) is based on modifying immuno-suppressant therapy. Patients are unlikely to benefit from antirejection treatment (ie, IV immunoglobulin or corticosteroids).
Emerging technologies
Overcoming blood group incompatibility
Until recently, transplants between donors and recipients with differing blood groups was considered to be impossible. However, techniques such as immunoadsorption (the removal of circulating antibodies) and the use of immunoglobulin and rituximab before transplantation can allow for successful transplantation of organs from a live donor.
Belatacept
Belatacept blocks CD28-mediated T-cell activation by binding CD80 and CD86 on antigen-presenting cells. Results so far show it to be non-inferior to ciclosporin as a means of preventing acute rejection and could reduce the rate of chronic allograft nephropathy [8].
Janus kinase inhibitors
Janus kinase (JK) inhibitors block cytokine production and might have a role in maintenance immunosuppression strategies [9]. In a six-month, phase II trial, 61 adults who had undergone a renal transplant were randomised to receive one of two dose levels of CP-690550 (a JK inhibitor) or tacrolimus in combination with basiliximab, mycophenolate andcorticosteroids [10].
The incidence of biopsy-proven acute rejection and the level of renal function at six months was comparable across all three groups with no graft loss, deaths or malignancies in any group. However, patients in the high-dose CP-690550 group developed a greater number of infections and were more likely to develop cytomegalovirus (CMV) disease and nephropathy associated with the BK virus — an incurable member of the polyomavirus family.
Conclusion
Rejection pathways are still not fully understood and thus immunosuppressive strategies and treatments are still evolving. The most ideal scenario is one of tolerance, where an allograft is accepted permanently by a recipient without the need for immunosuppression (which has occurred rarely in some individuals). However, mechanisms for how this might be achieved are yet to be discovered. At present, tailoring of immunosuppression to individual transplant recipients will improve the chances of graft and patient survival for all forms of solid organ transplantation.
Reena Popatis senior renal pharmacist at Barts andthe London NHS Trust.E: reena.popat@bartsandthelondon.nhs.uk
References
- Kapic E, Becic F, Kusturica J. Basiliximab mechanisms of action and pharmacological properties. Medicinski Arhiv 2004;58:373–6.
- National Institute for Health and Clinical Excellence. Immunosuppressive therapy for renal transplantation in adults. September 2004. www.nice.org.uk/ta85 (accessed 19 January 2010).
- Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulinversus basiliximab in renal transplantation. New England Journal of Medicine 2006;355:1967–77.
- Danovitch GM, editor. Handbook of kidney transplantation. 4th edition. Philadelphia: Lippincott Williams & Wilkins; 2004.
- Margreiter R, Klempnauer J, Neuhaus P, et al. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. American Journal of Transplantation2008;8:1480–5.
- Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. New England Journal of Medicine 2007;357:2562–75.
- Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renalallograft pathology: updates and future directions. American Journal of Transplantation 2008;8:753–60.
- Vincenti F, Larsen , Durrbach A. Costimulation blockade with belataceptin renal transplantation. New England Journal of Medicine 2005;353:770–81.
- Vincentia F, Kirk AD. What’s next in the pipeline. American Journal ofTransplantation 2008;8:1972–81.
- Hardinger KL, Koch MJ, Brennan DC. Current and futureimmunosuppressive strategies in renal transplantation. Pharmacotherapy2004;24:1159–76.