BSIP SA / Alamy
Summary
Osteoarthritis (OA) is the most common form of arthritis worldwide and is associated with increasing age and obesity. OA can affect weight-bearing and non-weight-bearing joints, and may involve single or multiple joints. Each of these types of OA has their own risk factors and there is currently no accurate risk tool for predicting the onset of OA.
There are no medical treatments proven to prevent or delay the onset of OA. However, non-pharmacological measures, such as weight loss, structured exercise programmes and various aids, can help to improve symptoms.
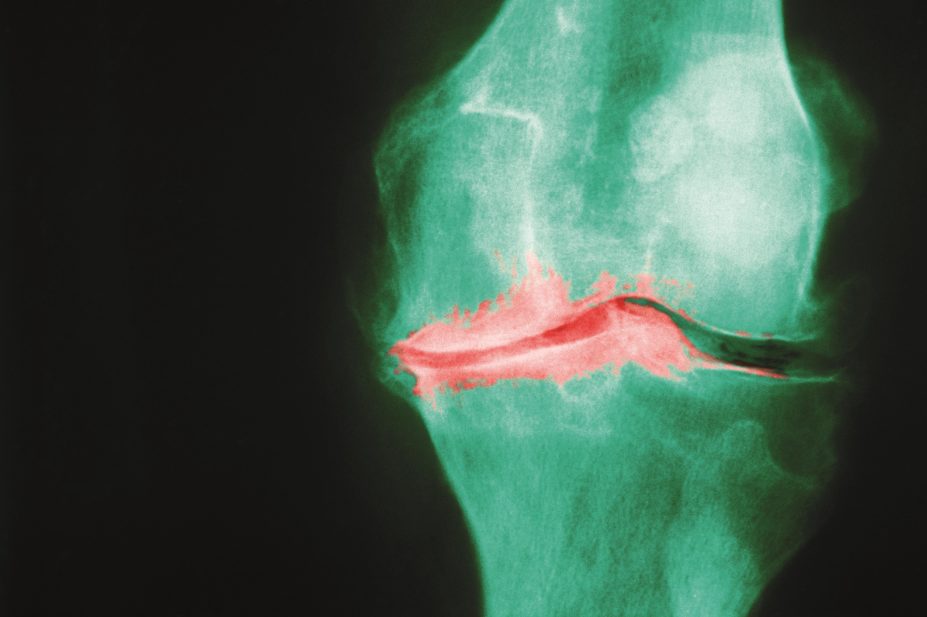
The diagnosis of OA is likely when an individual with OA risk factors presents with the combination of typical mechanical joint pain symptoms and joint examination findings of OA. X-rays may be needed if there is uncertainty about the diagnosis. Typical OA features seen on X-ray include joint space narrowing, bone spurs, subchondral sclerosis and subchondral cysts. Blood tests are not required for a diagnosis of OA, but may be performed to exclude other diseases such as psoriatic arthritis, rheumatoid arthritis and gout.
Osteoarthritis (OA) is a degenerative disorder of joints that most frequently affects the knee, hip, spine and small joints of the hand. It is the most common form of arthritis worldwide and causes significant pain and disability. There are 8.5 million people with OA in the UK and two million GP consultations per year are for painful OA[1]
.
More than two-thirds of people with OA are in constant pain. While most people with OA are taking treatment, more than one-third of those taking treatment feel it is not very effective or not effective at all[1]
.
OA is the eleventh highest contributor to global disability[2]
. One-third of people with OA either give up work, retire early or reduce the number of hours they work[1]
. Obesity and increasing age are important risk factors for OA, with symptomatic manifestations usually not occurring until middle age. Therefore, the burden of OA is destined to increase as the population ages and the prevalence of obesity increases[3]
.
OA may be classified as either primary or secondary OA. In primary OA the cause is unknown (idiopathic), while in secondary OA there is a link to a specific cause, such as previous injury to a joint, pre-existing congenital abnormality (congenital hip dysplasia) or inflammatory arthritis such as gout or rheumatoid arthritis (RA).
There are no medical treatments proven to prevent or delay the onset of OA. However, non-pharmacological measures, such as weight loss, structured exercise programmes and various aids, can help to improve symptoms in OA.
Pathophysiology
In healthy joints, two bones articulate with one another separated by shock-absorbing cartilage. Both the cartilage and the bone are important in dissipating the load placed through joints every day. When joints are subjected to large loads or impact, the cartilage and bone may be damaged.
Numerous repair mechanisms attempt to restore normal function within the damaged joint to ensure the joint continues to dissipate load correctly. This includes cell-mediated remodelling within the architecture of the cartilage and subchondral bone tissues[4]
. When the rate of damage exceeds the rate of repair, degeneration of the bone and cartilage ensues and the joint fails to effectively dissipate load.
This results in a cycle of biomechanical and biochemical degeneration, where the shock-absorbing cartilage is progressively destroyed, exposing the bone to greater load and leading to bone damage (bone marrow lesions)[5]
. This leads to further loss of cartilage, narrowing of the joint space (the space between the bones) and the overgrowth of bone (osteophyte formation), which causes hard lumps to develop around the joints.
Joint degeneration results in painful and tender inflammation of the synovial lining of the joint (synovitis) and swelling of the joint (effusion)[6]
. While the cartilage, bone and synovial lining are important tissues, the whole joint is involved in the pathogenesis.
Modern imaging techniques, such as magnetic resonance imaging (MRI), have established that the loss of cartilage, bone marrow lesions and synovitis are common in OA and are predictive factors for requiring a joint replacement[7]
. MRI-detected synovitis and bone marrow lesions are also associated with OA pain[8]
. Synovitis may be present in early cartilage changes to the joint, and there is cellular infiltration with macrophages, activated T and B cells and accompanying vascular proliferation. Inflammatory cytokine levels may also be elevated in the joint, but to a lesser extent than seen in RA.
Aetiology
There is no single cause of OA, and the exact aetiology of OA is unknown. A combination of factors increase the risk of developing OA. These include:
- Aged over 50 years
- Female sex
- Increased body mass index (BMI>25)
- Previous injury to the affected joint
- Laxity of joint ligaments
- Occupational or recreational use of the affected joint
- Family history.
OA can affect weight-bearing and non-weight-bearing joints, and may involve single or multiple joints. Each of these types of OA have their own risk factors and there is currently no validated risk tool for the quantitative prediction of developing OA.
Sex hormones may influence the development of hand OA among young women[9]
. Women younger than 40 years of age have a lower incidence of hand OA compared with men of the same age; however, over the age of 40 years there is a higher incidence in women. However, to date hormone replacement therapy (HRT) studies have not supported this hypothesis, as there was no reduction in hand OA in women who have received HRT. Further research is required to fully evaluate the relationship between female hormones and OA.
Genetic studies have shown that OA is genetically heterogenous, with common gene variants contributing only modestly to the risk of OA. However, studies have suggested there may be genetic variation in the causes of pain among people with OA. The SCN9A gene is responsible for three inherited human pain disorders (primary erythermalgia, paroxysmal extreme pain disorder and channelopathy-associated insensitivity to pain) and variation at this gene affecting peripheral pain thresholds have been implicated in various forms of chronic pain, including in people with OA[10]
.
Diagnosis
The diagnosis and subsequent management of OA is dependent on the type and number of joints affected (see below for how OA is identified in different joints).
OA is characterised by pain that is worse with activity and associated with only a short period of early morning stiffness; in contrast, RA commonly causes early morning stiffness lasting more than one hour.
Diagnosis is based on the combination of typical mechanical pain symptoms and physical joint findings of OA in an individual with risk factors. In rare circumstances, X-rays are taken if there is uncertainty. There is no diagnostic tool for OA that has been validated for use in routine clinical practice.
Studies and guidance on the management of OA often focus on a single joint being affected, such as the hip, knee or hand[11]
. However, individuals with OA may often present with pain in more than one joint. Pain associated with OA is typically gradual in onset unless the cause is traumatic. People with OA frequently present with muscle wasting around the affected joints caused by the patients avoiding movement (disuse atrophy); this is associated with greater pain severity[12]
.
Typical OA features seen on X-ray include joint space narrowing, bone spurs (osteophytes), subchondral sclerosis and subchondral cysts. Blood tests are not required for a diagnosis of OA, but may be performed to exclude other diseases such as psoriatic arthritis, RA and gout. A normal X-ray does not exclude the diagnosis of OA.
A differential diagnosis for OA is gout. Gout is commonly distinguished from OA by a flare period followed by complete remission between flares. However, long-term damage to joints associated with uncontrolled inflammation in gout (and RA) may ultimately result in the development of OA in the affected joint, making it difficult to distinguish the types of arthritis by symptoms alone. Furthermore, OA often co-exists with gout, resulting in chronic joint pain in the absence of active disease.
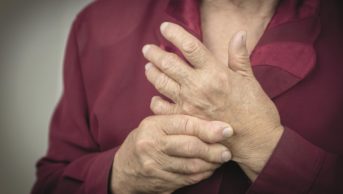
Symptomatic OA of the hand affects 8% of people aged 60 years or over, and 26% of women and 13% of men aged 70 years or over[13],[14]
. The most commonly affected joints are the distal and proximal interphalangeal joints (DIPs and PIPs), followed by the base of the thumb. People with hand OA experience significant difficulty with daily tasks such as gripping, writing, carrying heavy items and picking up small objects. Functional impairment may be as severe as that associated with people with RA.
The typical symptoms associated with hand OA include pain on activity, mild morning stiffness or stiffness following inactivity. This may be intermittent, and may affect one or many joints at any one time. There may be poor correlation between the severity of pain and severity of radiographic changes seen on X-ray. People who have multiple hand joints affected (polyarticular) may be at increased risk of also having knee and hip OA.
The hallmark features of hand OA are Heberden’s nodes and Bouchard’s nodes. Heberden’s nodes are hard, bony swellings occurring on the DIP joints at the end of the fingers. They are bone ‘overgrowths’ or osteophytes that form in articular cartilage as part of the joint repair mechanism. Bouchard’s nodes are less common in OA and are hard, bony or gelatinous cysts on the PIP joints.
Erosive hand OA can occur in the interphalangeal joints (IPJs). Erosive hand OA can have an abrupt onset with marked pain, functional impairment, stiffness, tissue swelling, redness and numbness. Bone erosions are typically found beneath the cartilage and a vicious cycle of cartilage and bone degradation may result in instability of the joint and impaired movement.
Hand OA can be diagnosed based upon the presence of typical symptoms and clinical findings. A hand X-ray can be used if there is any clinical uncertainty.
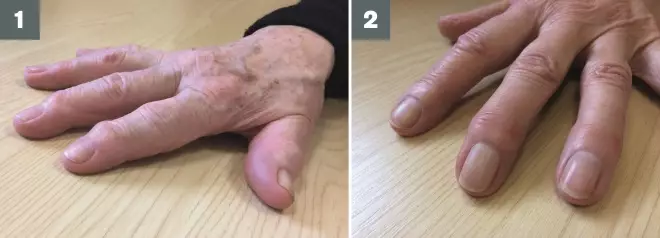
Source: Andrew Barr
1) Heberden’s nodes are hard, bony swellings occuring on the distal interphalangeal joints at the end of the fingers, and occur as part of joint repair.
2) Bouchard’s nodes are hard, bony or gelatinous cysts occuring on the proximal interphalangeal joints (the middle of the fingers); these are less common in OA than Heberden’s nodes.
Knee OA is the most common arthropathy to affect the knee joint, and the knee is the large joint most commonly affected by OA. People with knee OA typically experience activity-related pain, which is often worst towards the end of the day. The pain is relieved by rest and there is only mild morning or inactivity-related stiffness. The symptoms may be episodic and vary in severity. People may also describe a feeling of the knee joint ‘giving way’. In more advanced knee OA, the pain may become more persistent and may occur at rest and during the night.
Current guidance from the European League Against Rheumatism (EULAR) recommends that a diagnosis of OA of the knee can be made without the need for X-ray in people who are aged over 40 years with[11]
:
- Persistent pain
- Limited morning stiffness
- Reduced function
- One or more of crepitus (noise on movement); restricted movement; bony enlargement.
For people below the age of 40, the diagnosis of OA can be made with exactly the same approach, however the likelihood of OA is relatively reduced.
Hip OA is the second most common large joint OA[2]
. Risk factors include obesity, employment that involves lifting heavy objects, physical work such as farming, and certain sports activities such as running[3]
.
People with hip OA experience difficulty in moving their hip joints, which may be worse on awakening or after sitting in a chair for a period of time. The individual may encounter difficulty when putting their shoes and socks on, getting in and out of a car, or going up and down stairs. Pain related to hip OA can occur in different locations than the thigh area, including the groin, buttocks and knees. People may therefore not realise that the pain originates from their hip joint. The intensity of the pain increases when the hip joint is moved.
As with other forms of OA, the diagnosis is made based upon the presence of typical symptoms and clinical findings but X-rays may be helpful. Additional imaging, such as CT and MRI, may be performed if the diagnosis is unclear. Blood tests are not required to confirm the diagnosis, but may be performed to exclude infection or an inflammatory arthropathy.
Prognosis
The prognosis of OA can range from a stable good quality of life with manageable pain and an active lifestyle, to a progressive deterioration in quality of life, persistent severe pain and limited mobility and function. This is dependent on many factors, including which joints are involved.
For knee OA, pain and function outcomes are better with greater muscle strength and aerobic exercise, improved mental health, social support and self-management coping strategies. Worse pain and function is associated with increased age, BMI and knee pain severity[15]
.
For hip and hand OA, the prognosis is less clear. Unlike non-weight bearing joints, around a quarter of people with hand OA describe a significant reduction in pain in the long term[16]
.
Tina Hawkins MRPharmS Ipresc is an advanced clinical pharmacist in rheumatology at Leeds Teaching Hospitals NHS Trust, and Andrew J Barr MBBS MRCP is a clinical research fellow at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds and NIHR Leeds Musculoskeletal Biomedical Research.
The authors have no interests to declare. Andrew Barr’s work is funded by Arthritis Research UK (grant 20154).
References
[1] Care A. OANation 2012 report. Available at: www.arthritiscare.org.uk.
[2] Cross M, Smith E, Hoy D et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73(7):1323–1330.
[3] Bijlsma JW, Berenbaum F & Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet 2011;377(9783):2115–2126.
[4] Goldring MB & Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann New York Academy Sciences 2010;1192:230–237.
[5] Suri S & Walsh DA. Osteochondral alterations in osteoarthritis. Bone 2012;51(2):204–211.
[6] Sellam J & Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Rev Rheumatol 2010;6(11):625–635.
[7] Roemer FW, Kwoh CK, Hannon MJ et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology 2015;274(3):810–820.
[8] Yusuf E, Kortekaas MC, Watt I et al. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheumatic Dis. 2011;70(1):60–67.
[9] Zhang W, Doherty M, Leeb BF et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheumatic Dis. 2009;68(1):8–17.
[10] Thakur M, Dawes JM & McMahon SB. Genomics of pain in osteoarthritis. Osteoarthritis and Cartilage 2013;21(9):1374–1382.
[11] Zhang W, Doherty M, Peat G et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheumatic Dis. 2010;69(3):483–489.
[12] Ruhdorfer A, Wirth W, Hitzl W et al. Association of thigh muscle strength with knee symptoms and radiographic disease stage of osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care & Research 2014;66(9):1344–1353.
[13] Dillon CF, Hirsch R, Rasch EK et al. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and Nutrition Examination Survey, 1991–1994. Am J Physical Med Rehab 2007;86(1):12–21.
[14] Zhang Y, Niu J, Kelly-Hayes M et al. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156(11):1021–1027.
[15] van Dijk GM, Dekker J, Veenhof C et al. Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheumatism 2006;55(5):779–785.
[16] Kloppenburg M & Kwok WY. Hand osteoarthritis — a heterogeneous disorder. Nature Rev Rheumatol 2012;8(1):22–31.