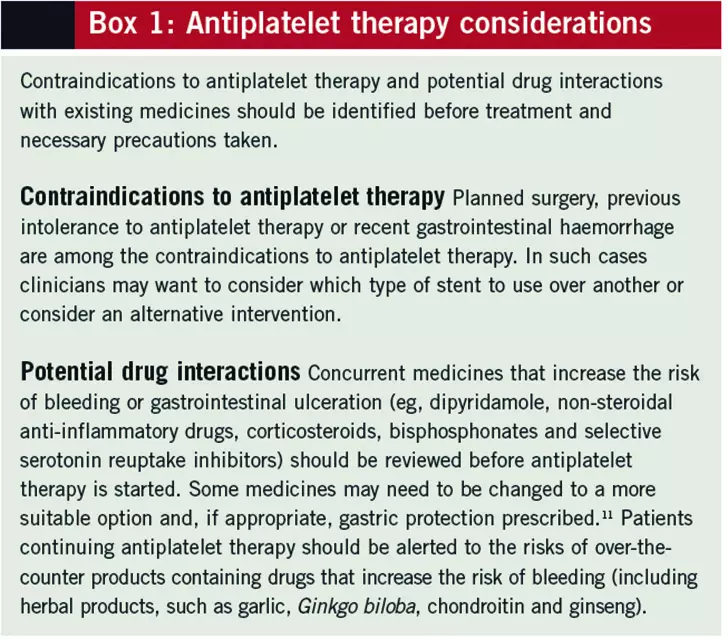
This content was published in 2013. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
Complications can occur during, shortly after or at any time following percutaneous corornary intervention (PCI). Procedural complications such as contrast-induced nephrotoxicity, metabolic acidosis, restenosis and bleeding from the access site can jeopardise the health of the patient and require appropriate management.
Antiplatelet therapy is vital after stent implantation to prevent in-stent thrombosis. Choice, number and duration of antiplatelets for patients post-PCI depend on the reason for intervention, the type of stent implanted and any pre-existing conditions.
The past decade has seen major advances in the treatment of coronary heart disease through the use of percutaneous coronary interventions (PCIs). Operator techniques have been refined, equipment has improved and stent technology has advanced to produce better clinical outcomes.
With advancing practice boosting clinical benefit from PCI, care should be taken to prevent complications associated with these interventions. The techniques used during angiography and stent insertion can put the patient at risk during and shortly after the procedure. Appropriate preparation can ensure that the risks associated with PCI can be minimised. Likewise, for patients having stents inserted the importance of adequate ongoing antiplatelet therapy cannot be underestimated.
Procedure-related complications
Contrast-induced nephrotoxicity
Radio-opaque dyes are used as contrast media to define narrowing in the coronary arteries as part of the angiographic procedure. The dyes are excreted almost exclusively via glomerular filtration by the kidneys. The risk of contrast-induced nephrotoxicity (CIN) is increased by a number of factors, including the amount of dye administered, older age, hypotension, diabetes mellitus and renal impairment.1
Although the clinical benefit of using sodium bicarbonate or N-acetylcysteine (NAC) to prevent CIN has been questioned in a number of meta-analyses,2,3 a small number of randomised controlled trials have suggested they do help (although the extent of benefit is difficult to quantify). Nonetheless, sodium bicarbonate and NAC cause negligible harm to patients and so they are often used routinely in the prevention of CIN — particularly for patients with renal impairment.
It is agreed that adequate hydration is essential4 and, if clinically possible, patients with moderate renal impairment should be given intravenous fluids to encourage excretion of the dye.
Metabolic acidosis
Patients with diabetes mellitus who take metformin are at risk of metabolic acidosis when administered contrast media.5 Although manufacturers of metformin suggest the drug should be withheld before, and for 48 hours after, administration of contrast media (the length of time depending on renal function), the European Society of Urogenital Radiology and the Royal College of Radiologists have produced alternative guidance.4,6 They recommend that patients who have an estimated glomerular filtration rate (eGFR) of >60ml/min/1.73m2 do not need to withhold metformin and those who have an eGFR of 30–60ml/min/1.73m2 should withhold it for 48 hours before and after the administration of contrast media.
Bleeding
There are two arterial access points that are used to perform angiographic procedures. In the past, the femoral artery has been the preferred option because it is easier for the operator to manipulate a catheter into position through this route. However, using this option carries an increased risk of bleeding from the access site. Patients need to remain immobile for a period after the procedure, to allow time for the artery to close, and this can be difficult in practice.
In a randomised study, a comparison of the radial and femoral access routes for angiographic procedures for patients with acute coronary syndrome (ACS) suggested there was no difference in the occurrence of major adverse coronary events between the two groups; however, more vascular-related complications occurred using the femoral route.7
In recent years, there has been a shift towards using radial access to perform emergency angiographic procedures following suggestions that this route is safer and offers better long-term outcomes. A common problem with using radial access is radial artery spasm, which can prevent catheter progression through the artery. This can be avoided by using a combination of verapamil, glyceryl trinitrate and heparin (often referred to as a radial cocktail), administered just before the procedure.8
Stent-related complications
Restenosis
Restenosis is the gradual narrowing of the artery lumen after PCI. Early restenosis can occur within 24 hours of traditional balloon angioplasty following recoil of the balloon. This type of restenosis can be avoided through the use of stent technology.
Late restenosis occurs when smooth muscle cells proliferate around a stent some four to six months after the procedure. Termed in-stent restenosis, this can lead to intimal hyperplasia and restenosis within the stented segment of artery9 and can require further intervention if symptoms of coronary artery disease return. Arteries that are of small lumen diameter (<3mm), saphenous vein grafts and longer lesions (>15mm) are more likely to succumb to late restenosis.10
In-stent thrombosis
A serious complication of coronary artery insertion is in-stent thrombosis (IST). This is the sudden formation of a blood clot in the artery at the site of the stent. It can be caused by activation of the clotting system, usually through platelet activation, in response to the detection of a foreign body in the artery (the metal framework of the stent before complete endothelialisation).
Although the expected occurrence of IST is relatively low, it is associated with a high risk of death. Factors that can lead to IST include:
- Under-deployment of the stent, ie, the stent does not fully embed into the artery lumen
- Temporary cessation of antiplatelet therapy (due to surgery or non-adherence)
- Hyporesponsiveness to antiplatelets
Preventing in-stent thrombosis
Loading doses of antiplatelets are given before PCI to prevent clots occurring during the procedure. However, continuation of antiplatelet therapy is also needed to reduce the risk of IST after stent placement. Choice of antiplatelet depends on existing conditions and whether the procedure was elective or done in an emergency. This is discussed in more detail in the accompanying article.
After patients have completed the necessary duration of dual antiplatelet therapy post-PCI (as discussed below) they will be required to continue one antiplatelet long term. This is usually aspirin but in some cases clopidogrel is continued, such as in peripheral vascular disease or if a patient has previously had a cerebral vascular accident.
Dual antiplatelet therapy
The duration of dual antiplatelet therapy following stent insertion depends on the type of PCI performed and presence of any conflicting factors around antiplatelet use (see Boxes 1 and 2). For drug-eluting stents, endothelialisation of the stent is delayed. Although this prevents in-stent restenosis, it does mean the metal framework of the stent is exposed for longer — prolonging the need for dual therapy.

Practice in the UK typically reflects the recommendations made by the European Society of Cardiology guidelines. These suggest that for elective procedures dual therapy should be continued for one month after bare metal stent implantation and for six to 12 months after drug-eluting stent implantation. For patients presenting with ACS who undergo emergency PCI, dual therapy is suggested for one year irrespective of the type of stent used.15 As above, once patients have completed this course of dual therapy a single antiplatelet drug is recommended to be continued for life.
Dual antiplatelet therapy and anticoagulation
For patients with a compelling indication for long-term anticoagulation the consensus from the ESC is that, where appropriate, bare metal stents, balloon angioplasty or coronary artery bypass graft should be preferred over drug-eluting stents. This restricts the duration of triple therapy (ie, two antiplatelets and an anticoagulant) to one month16 and so reduces the amount of time the patient is at an increased risk of major bleeding.
If a drug-eluting stent is used then the duration of triple therapy required ranges from three to six months depending on the type of stent used (ie, three months for stents containing sirolimus or sirolimus analogues and six months for those containing paclitaxel or other similar drugs). After this, one antiplatelet plus anticoagulation is suggested for the remainder of the year and then anticoagulation on its own continued long term.16
References
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast induced nephropathy after percutaneous coronary intervention. Journal of the American Journal of Cardiology 2004;44:1393–9.
- Zagler A, Azadpour M, Mercado C, et al. N-acetylcysteine and contrast-induced nephropathy: A meta-analysis of 13 randomized trials. American Heart Journal 2006;151:140–5.
- Kunadian V, Zaman A, Spyridopoulos I, et al. Sodium bicarbonate for the prevention of contrast induced nephropathy: A meta-analysis of published clinical trials. European Journal of Radiolology 2011;79:48–55.
- Royal College of Radiologists. Standards for intravascular contrast agent administration to adult patients. 2010.
- Bui KL, Horner JD, Herts BR, et al. Intravenous iodinated contrast agents: Risks and problematic situations. Cleveland Clinic Journal of Medicine 2007;74:361–7.
- European Society of Urogenital Radiology. Guidelines on constrast media. 2008. www.esur.org (accessed 16 January 2013).
- Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography andintervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011;377:1409–20.
- Frangos C, Noble S. How to transform you into a radialist: Tips and tricks. Cardiovascular medicine 2011;14:315–324.
- Maisel WH, Lakey WK. Drug-eluting stents. Circulation 2007;115:e426–7.
- National Institute for Health and Clinical Excellence. Drug eluting stents for the treatment of coronary artery disease. July 2008. www.nice.org.uk/ta152 (accessed 16 January 2013).
- Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous coronary interventions. European Heart Journal 2005;26:804–47.
- Spertus JA, Kettelkamp R, Vace C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement. Circulation. 2006;113:2803–9.
- Hospital Pharmacist Newsletter. United Kingdom Clinical Pharmacy Association launches clopidogrel card for patients. Pharmaceutical Journal 2006;October suppl:S3.
- Antoniou S, Rothman MT. Stent thrombosis (letter response) BMJ 2007;334:57.
- Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. European Heart Journal 2010;31:2501–55.
- Lip GY, Huber K, Andreotti F, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting. European Heart Journal 2010;31:1311–8.