Shutterstock.com
After reading this article, you should be able to:
Congenital heart disease (CHD) is defined as a problem in the development of the heart that is present at birth1. There are many different types of CHD — for example, hole(s) between the atria or ventricles, valve defects or the narrowing of the aorta2.
Inherited cardiac conditions (ICC) are disorders caused by genetic variants affecting the heart and its electrical circuits or blood vessels3. Examples of these conditions include long QT syndrome4, Marfan syndrome5 and cardiomyopathies6. ICC conditions will not be discussed in this article; however, further information can be found on the British Congenital Cardiac Association website.
CHD is one of the most common types of birth defects and is estimated to affect almost 1 in 100 live babies born in the UK1. However, the true incidence rate may be higher, given that miscarriages, still births and terminations are not included in this statistic.
Pharmacists across sectors will encounter patients with CHD at different stages of their lives, which include maternity services where infants with CHD are delivered, neonatal and paediatrics care settings7, primary care, transitional care from paediatrics to adult services, palliative care, as well as adult respiratory or cardiology clinics.
The efforts of specialist CHD teams have driven significant developments in patient care over recent years — as more CHD patients are surviving into adulthood — with an estimated 2.3 million people living with CHD in the UK8.
How the heart is formed in utero
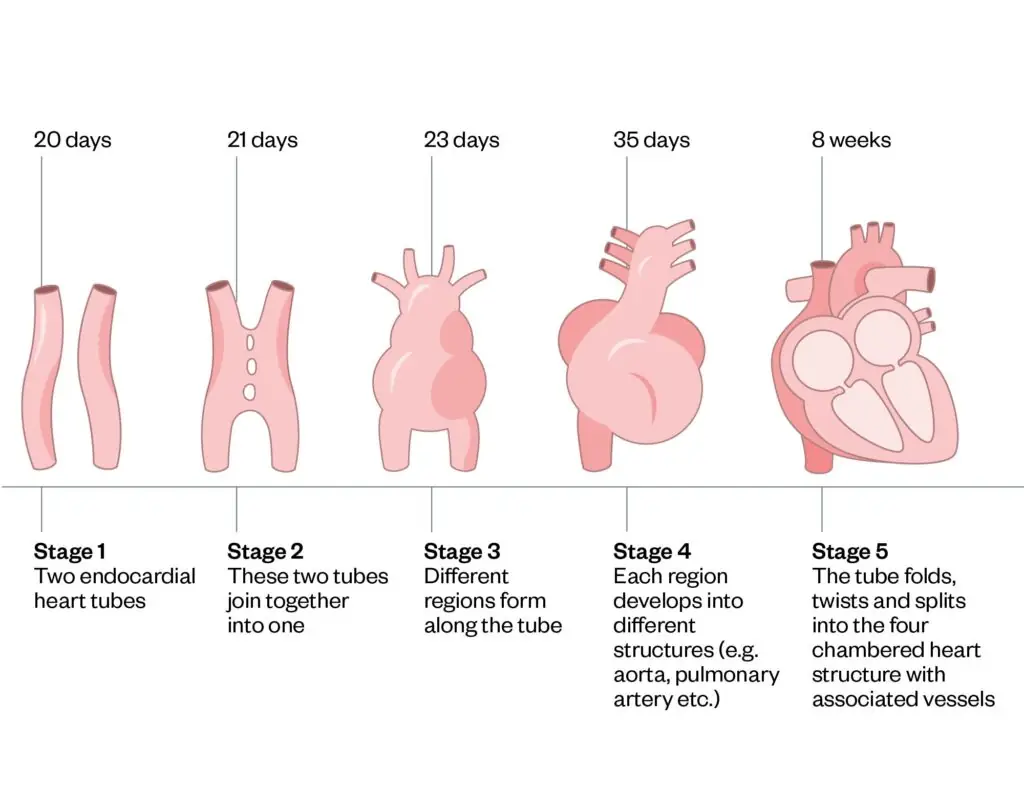
The heart is the first organ to function in the embryo, with the development of the aortic and pulmonary vessels beginning within the first month of foetal life and completing by eight weeks’ gestation9. By four weeks of foetal life, the heart beats for the first time and is fully functional by weeks seven or eight10. Understanding the heart’s formation during foetal development helps explain the origins of some types of CHD, such as the presence of hole(s) in the heart at birth, and can pinpoint when in foetal development a problem may have occurred (see Figure 1)10.
Figure 1: The development of the heart
Causes
Although there are several contributory factors that have been identified to increase the risk of CHD, most CHD is idiopathic (i.e. where an obvious cause has not been found)1. Some of the contributing factors associated with an increased risk of CHD include smoking or alcohol consumption, genetic syndromes or a family history of CHD in first-degree relatives11.
There are some medicines that are known to increase the risk of CHD in babies when taken by the mother during pregnancy, which include phenytoin, lithium, amphetamines, warfarin, oestrogen or progesterone12. These medications should be avoided wherever possible during pregnancy, with suitable counselling for those of childbearing age. In addition, safety alerts are required for some medicines — for example, the use of sodium valproate in women and girls13.
CHD is often linked to various genetic and chromosomal conditions, with around 30% of newborns with chromosomal abnormalities affected by CHD14.
All pregnant mothers in the UK are offered a blood test between 11 to 14 weeks’ gestation15, which estimates the probability of the baby having a trisomy. Trisomy is a genetic condition where there is an additional copy of a chromosome, so a patient will have a total of 47 rather than 46 chromosomes. Trisomy 13 (i.e. Patau syndrome)16, Trisomy 18 (i.e. Edwards syndrome)17 and Trisomy 21 (i.e. Down’s syndrome)18 are all linked to cardiac defects.
If the probability of a baby having a trisomy is found to be high, the mother is offered an amniocentesis15. Amniocentesis is a procedure that inserts a needle into the uterus to extract amniotic fluid to withdraw cells to determine if the foetus has one of these genetic syndromes; however, this does carry a risk of miscarriage, which is estimated to be 1 in 200 women15.
Other genetic conditions that are associated with CHD include DiGeorge syndrome19 and Turner syndrome20.
Where are patients likely to present and how is the condition identified?
Many congenital heart defects can be detected before birth during routine antenatal ultrasound screening, which is typically at the 20-week scan15. This anomaly scan involves a sonographer scanning the baby and checking all structures for potential abnormalities21.
For the heart, the foetal cardiac protocol includes assessing the position of the heart within the chest (i.e. situs), examining the four chambers, the outflow tracts (i.e. aorta and pulmonary artery) and the three vessels (i.e. ductus arteriosus, transverse aortic arch and superior vena cava)21.
If any concerns about the heart are highlighted, the mother is referred to a foetal cardiologist for more detailed echocardiography22. Some pregnant mothers may be directly referred to a foetal cardiologist, owing to a known increased risk of CHD, such as if the mother has diabetes, if a foetal arrhythmia has been detected, if a parent has CHD or if the mother has been exposed to rubella23.
Some CHD is not detected antenatally but is identified at the newborn baby check. CHD is found by listening to the baby’s heart and discovering a murmur or poorly palpable femoral pulses, which may indicate a problem with the aorta and further investigation or urgent treatment is needed23.
Some babies may present with symptoms suggestive of CHD that have be identified by parents, GPs or health visitors. These symptoms include poor weight gain — sometimes termed as ‘failure to thrive’ —symptoms of heart failure, such as sweating or tachycardia, heart murmurs or simply parental concern that something is ‘just not right’1.
Occasionally, depending on the type of CHD, some patients present for the first time via accident and emergency, with symptoms such as cyanosis (i.e. blue colour of the skin), tachycardia, shortness of breath or tachypnoea (i.e. fast breathing). The severity and urgency of CHD will very much depend on the type of CHD the patient has. Some babies are not symptomatic at all. CHD can be identified in the first few hours of life, days, weeks, months or even years later1,2.
Tests used to diagnose CHD
Figure 2: Showing various tests used to aid in the diagnosis of congenital heart disease1–3,16–20,24

Common types of congenital heart disease
The following types of CHD account for the majority of heart defects and anomalies that are observed2.
Atrial septal defect
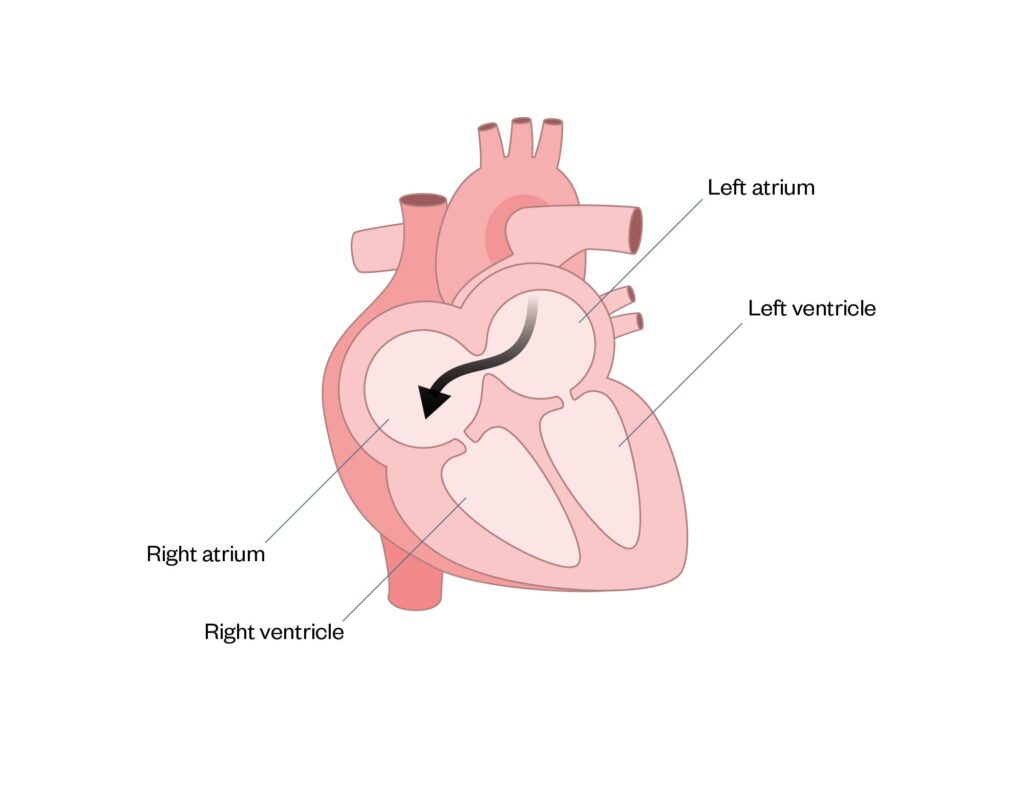
An atrial septal defect (ASD) is a hole between the atrial septum, facilitating mixing of blood between the left and right atrium (see Figure 3). If left untreated, pulmonary hypertension will develop in adulthood, owing to increased pressure to the lungs. If small, an ASD may close spontaneously; otherwise, it requires closure using a device fitted during a cardiac catheterisation procedure, or using a surgical patch to seal the hole, which requires open heart surgery25.
Figure 3: Atrial septal defect
Ventricular septal defect
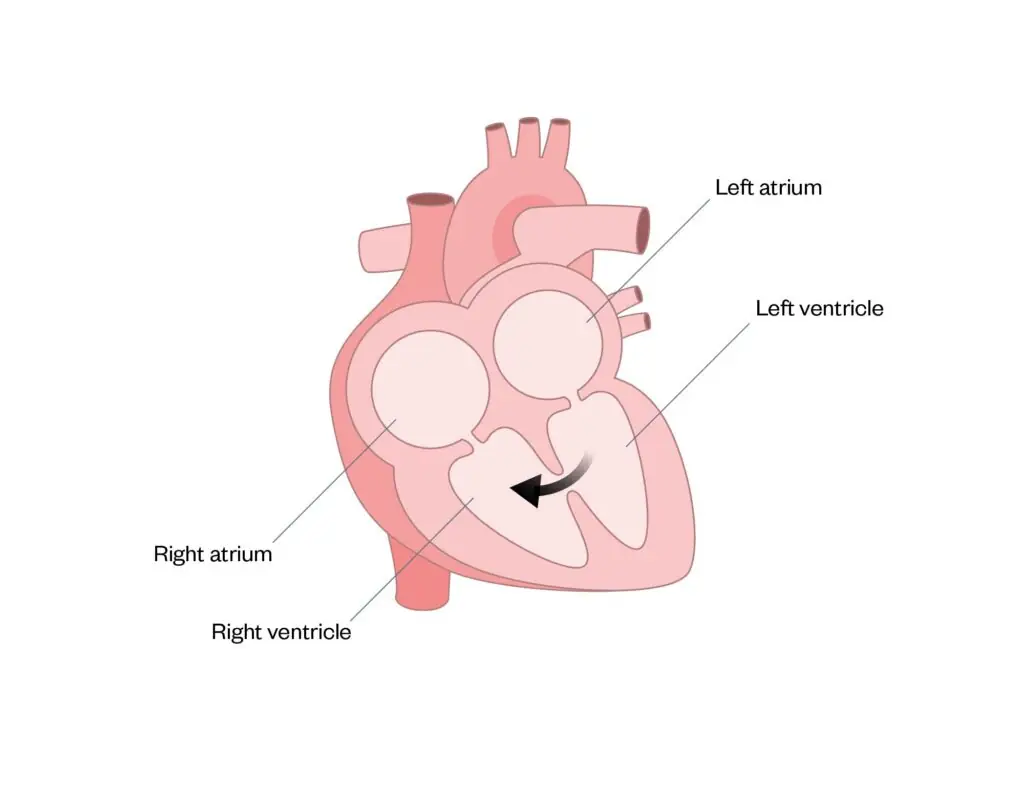
A ventricular septal defect (VSD) is where there is one or more hole(s) between the ventricles, with mixing of blood between the left and right ventricle (see Figure 4). VSDs can vary in size and position and may be part of a more complex defect. They cause increased blood flow to the lungs and, if left untreated, pulmonary hypertension will occur, making the heart work harder. If small enough, a VSD may close naturally; otherwise, treatment is similar to ASDs, using either a device or patch to close the hole26.
Figure 4: Ventricular septal defect
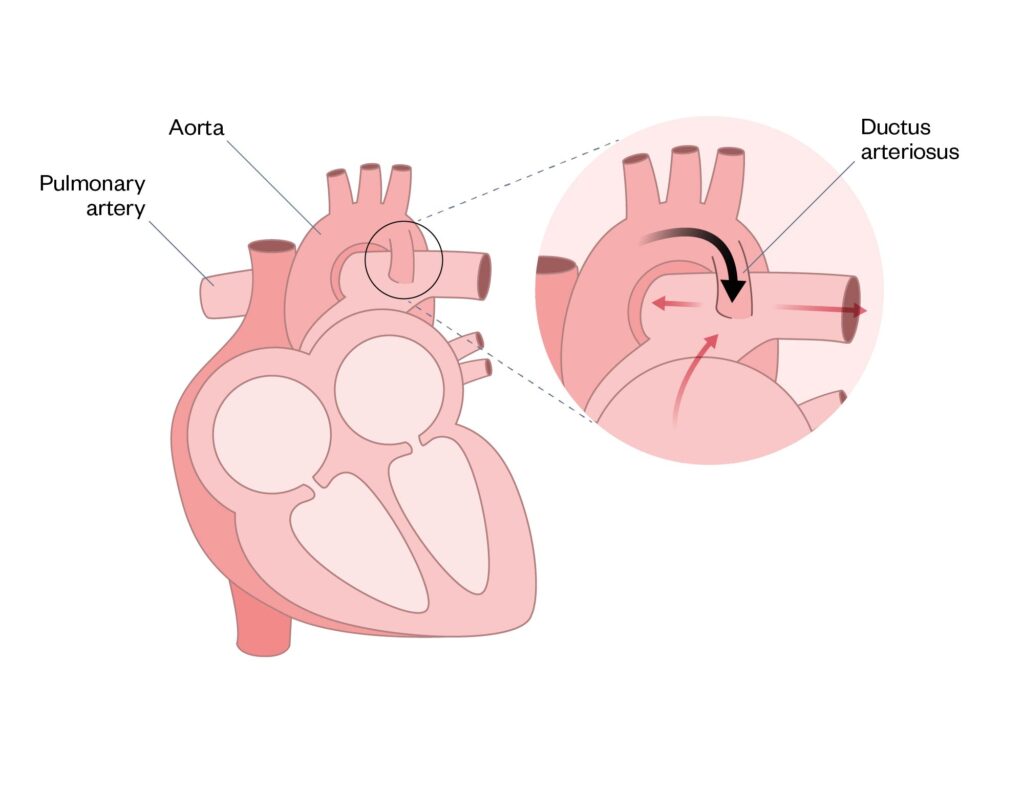
Patent ductus arteriosus
The patent ductus arteriosus (PDA) is a blood vessel that connects the main pulmonary artery to the descending aorta. It is critical for foetal circulation because the PDA allows blood flow to bypass the lungs (see Figure 5). The ductus arteriosus normally closes spontaneously shortly after birth; however, if this process does not occur, it is termed a PDA. PDAs are common in premature babies — for more information, see ‘Prematurity: managing associated complications’.
If the PDA is large and left untreated, damage to the lungs and heart can occur. There are several methods to close a PDA: medical treatment with paracetamol, coil closure using cardiac catheterisation, or surgical ligating (i.e. tying it off). For some presentations of CHD, it is helpful to maintain an open ductus arteriosus, which can be achieved using a continuous prostagladin infusion (see Coarcation of the aorta below)27.
Figure 5: Patent ductus arteriosus
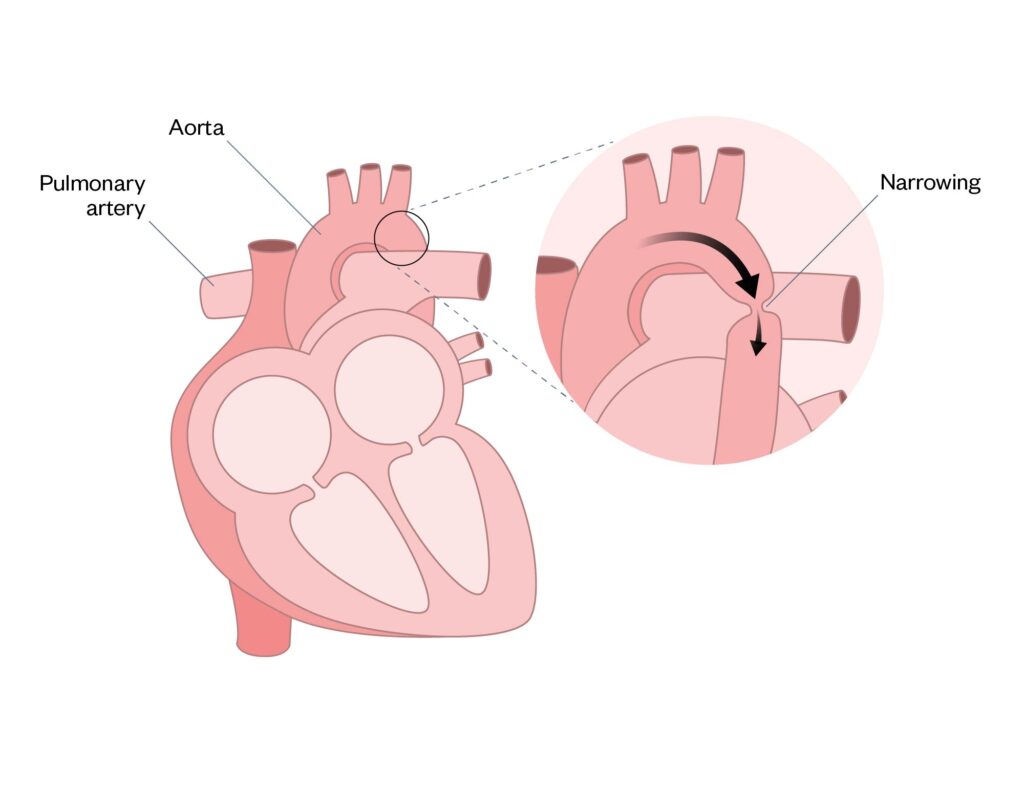
Coarctation of the aorta
Coarctation of the aorta (CoA) is a narrowing, or pinching, of the aorta with differing degrees of severity (see Figure 6). Keeping the ductus arteriosus open is advantageous and can be lifesaving, owing to it facilitating improved systemic blood flow until surgical correction of the CoA can occur. In addition, CoA is associated with an increased risk of necrotising enterocolitis (NEC). For more information on NEC see ‘Gastrointestinal complications associated with prematurity‘28–30.
Figure 6: Coarctation of the aorta
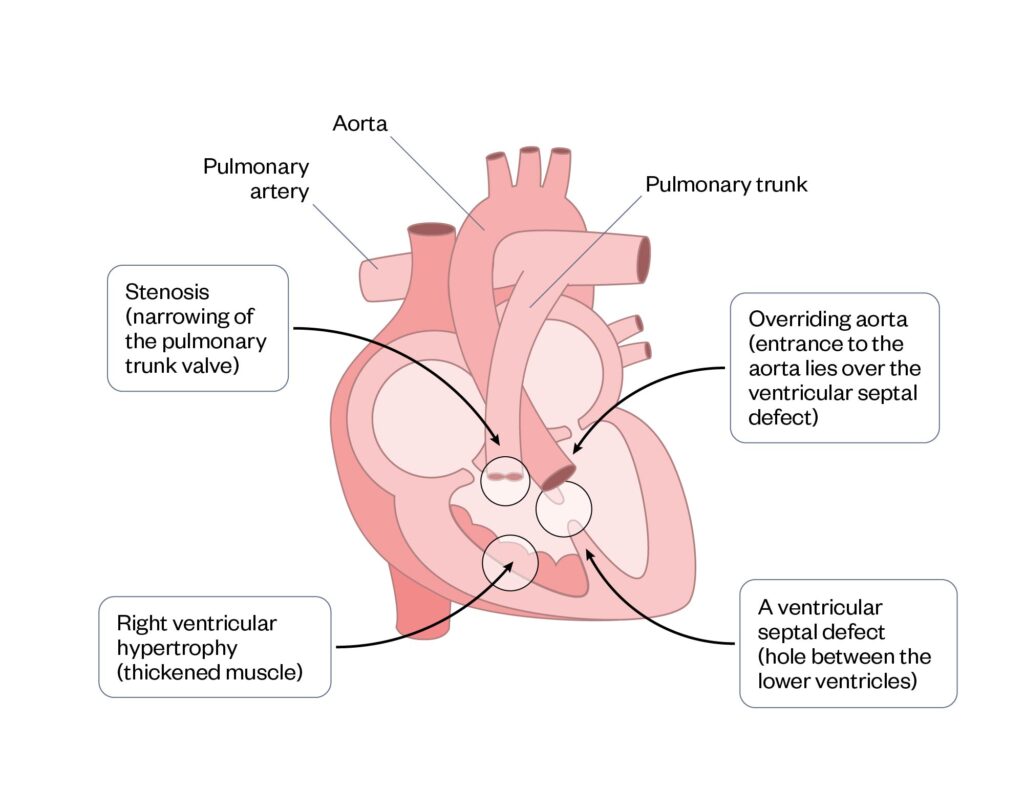
Tetralogy of Fallot
Tetralogy of Fallot (ToF) is a condition that comprises four distinct cardiac defects (see Figure 7). Children with ToF may present with severe cyanosis called ‘hypercyanotic spells’, which is a medical emergency and can be fatal. Reduced oxygen saturations and hypercyanotic spells will escalate the need for earlier surgical repair31. Surgical repair involves closure of the ventricular septal defect, removal of muscle from the right ventricle and pulmonary artery enlargement31.
Figure 7: Tetralogy of Fallot
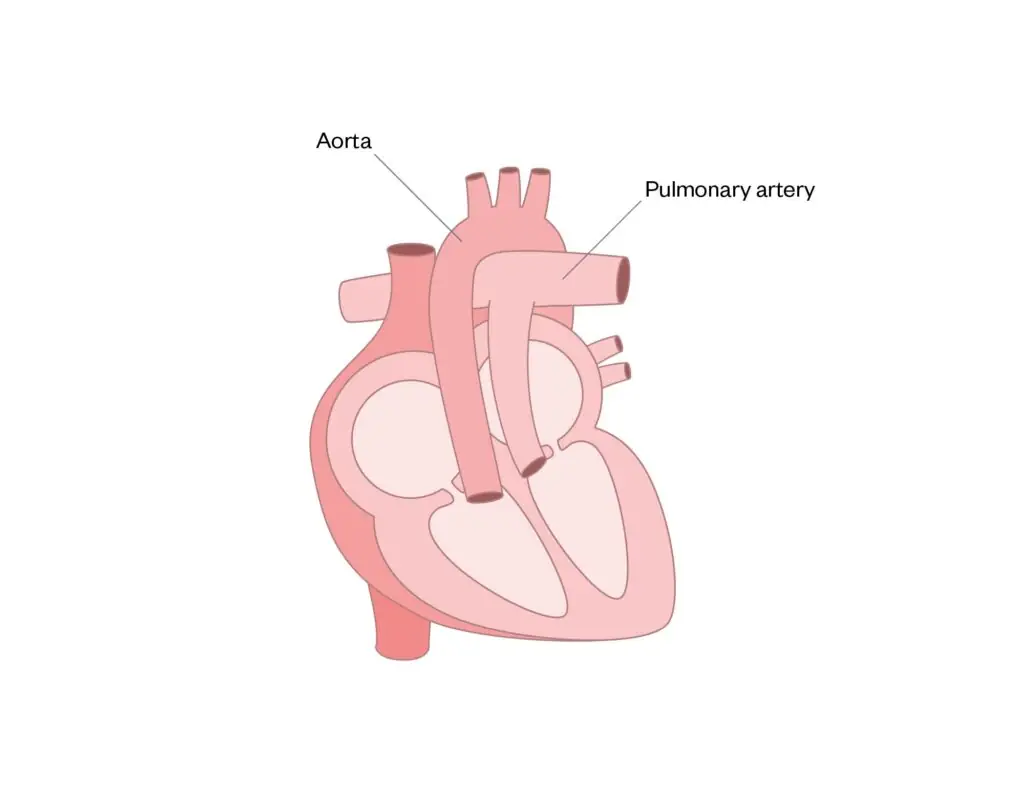
Transposition of the great arteries
The connections of the pulmonary artery and aorta are reversed, so that the pulmonary artery joins to the left ventricle and the aorta joins to the right ventricle (see Figure 8).
Surgical correction, which is called the ‘switch procedure’, involves switching the vessels to their normal positions, ensuring that valves are competent32.
Figure 8: Transposition of the great arteries
Atrio-ventricular septal defect
Atrio-ventricular septal defect (AVSD) is a common valve that can be classified as partial or complete, which is commonly seen in Down’s syndrome patients (see Figure 9)18,33.
Figure 9: Atrio-ventricular septal defect

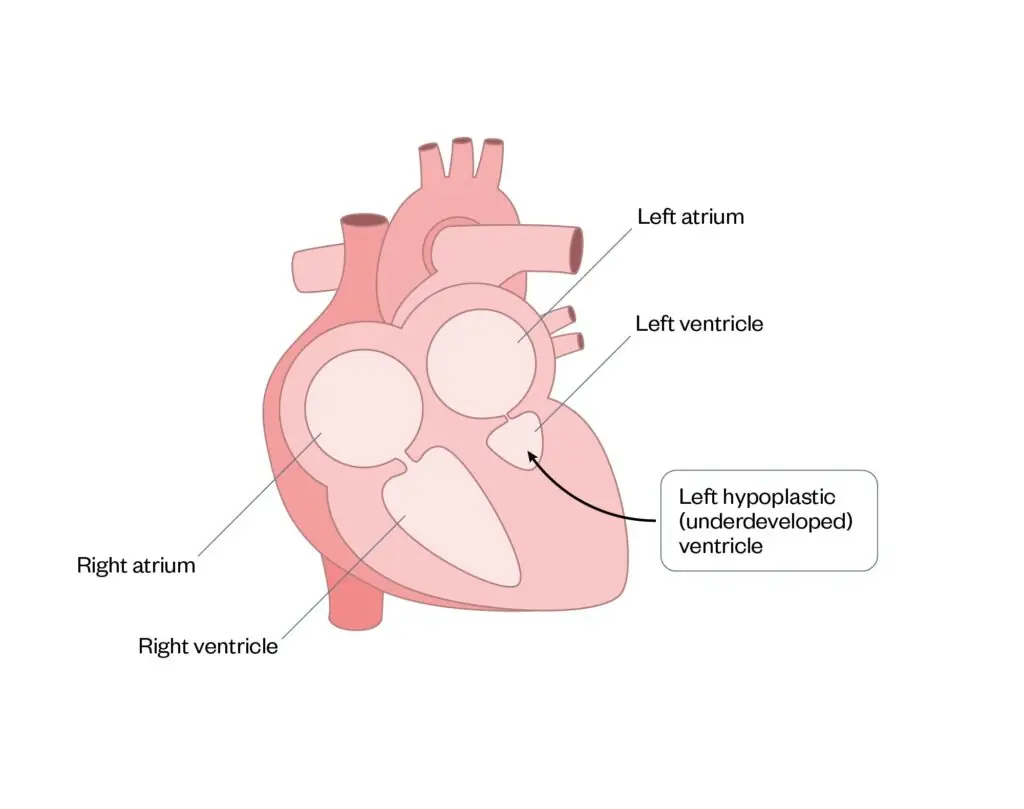
Hypoplastic left heart syndrome
Hypoplastic left heart syndrome is a condition where the left ventricle is underdeveloped and is smaller than usual, so it cannot support adequate systemic circulation (see Figure 10)34.
Figure 10: Hypoplastic left heart syndrome
Medication
When deciding on the pharmacological management of CHD, understanding of the anatomy, foetal heart development, the possible genetic diagnosis and the surgical options available is necessary. The indication of the medication may be different for each cardiac anomaly and influence the maximum dosages used35.
Guidelines are available for the management of CHD for adults from the European Society of Cardiology and the American College of Cardiology and American Heart Association36,37. Neonatal and paediatric guidelines are not as comprehensive but are evolving.
Medication can control symptoms preoperatively until surgical correction and give the patient sufficient time to reach a necessary surgical weight, readying the patient for the next-staged surgical procedure. In addition, medication may be stopped postoperatively after surgical correction or new medication is started for potential known complications, such as an arrhythmia or a clot1,2,35–37.
Based on the clinical needs of the patient, medications are given for palliative indications, for symptom management or control or when surgical options have been exhausted or are not appropriate38.
Table 1 shows just some examples of the medication that may be used at various points in a patient’s neonatal and paediatric CHD journey35.
Table: Different classes of medications and some examples of when they are used for congenital heart disease patients
Several medications highlighted in Table 1 are commonly used in adult patients; however, there are some additional considerations required when considering neonatal and paediatric patients.
Please see Box for a list of resources on medicines use in neonates and children to refer to for more information.
Box: Resources from The Pharmaceutical Journal on medicines use in neonates and children
Specifically, some of the practical considerations for medications used by neonatal and paediatric congenital heart disease patients include:
- Where do the parents or carers continue to get their medication supplies from? For example, if rivaroxaban is prescribed, what is the formulary status, will it come from GP, hospital or homecare provider?;
- Where will the monitoring of the medication take place? Blood tests for renal function for captopril may not be available at the patient’s local GP surgery, so the patient may need to travel to their local hospital;
- Some medications are titrated to effect (e.g. diuretics; furosemide and spironolactone);
- Consider the frequency of the medication — for example, if captopril is prescribed for a school-aged child at three-times-per-day dosing, perhaps converting to enalapril for ease of administration would be beneficial;
- Is the medication is contraindicated for children? Aspirin use is restricted for patients aged under 16 years, owing to the risk of Reye’s syndrome; however, for children at risk of clots, the benefits of low-dose aspirin in reducing blood clots far outweigh the risk of your child developing Reye’s syndrome;
- Can a child swallow a tablet? Is there an alternative dosage form available suitable at the correct dosage? (e.g. Entresto [Sacubitril/Valsartan; Novartis Pharmaceuticals] that is available in granules as well as tablets)39,40.
Conclusion
This article has outlined the basics of CHD: how common it is; some of the causes; how it can be diagnosed; awareness of the some of the different types; some of the medications used; and where to find further information to develop learning.
Signposting
There is a wealth of information available through charities, for both families and healthcare professionals, these include:
Further reading
If you would like to develop your learning, complete the Health Education England e-learning programme on the learning hub.
There are a few documents and events that have shaped, changed and continue to the develop the CHD journey:
Acknowledgements
A huge thanks to Teresa Brooks and Nicola Husain for their unwavering support for this article.
- 1.Congenital heart disease. NHS. 2021. https://www.nhs.uk/conditions/congenital-heart-disease/
- 2.Understanding your congenital heart condition. British Heart Foundation. https://www.bhf.org.uk/informationsupport/conditions/congenital-heart-disease
- 3.Inherited Cardiac Conditions. The British Congenital Cardiac Association. https://www.bcca-uk.org/inherited-cardiac-conditions
- 4.Long QT syndrome. NHS. 2025. https://www.nhs.uk/conditions/long-qt-syndrome/
- 5.Marfan Syndrome. The Marfan Foundation. https://marfan.org/conditions/marfan-syndrome/
- 6.Ciarambino T, Menna G, Sansone G, Giordano M. Cardiomyopathies: An Overview. IJMS. 2021;22(14):7722. doi:10.3390/ijms22147722
- 7.Newborn health. World Health Organization. https://www.who.int/westernpacific/health-topics/newborn-health#tab=tab_1
- 8.Adult congenital heart disease. British Heart Foundation. https://www.bhf.org.uk/informationsupport/conditions/adult-congenital-heart-disease
- 9.Development of the Heart. Oregon State University. https://open.oregonstate.education/aandp/chapter/19-5-development-of-the-heart/
- 10.Tan CMJ, Lewandowski AJ. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn Ther. 2019;47(5):373-386. doi:10.1159/000501906
- 11.Congenital Heart Defects – Causes and Risk Factors . National Heart, Lung, and Blood Institute. 2022. https://www.nhlbi.nih.gov/health/congenital-heart-defects/causes
- 12.Zierler S. Maternal drugs and congenital heart disease. National Library of Medicine . Published online February 1985. https://pubmed.ncbi.nlm.nih.gov/3881709/
- 13.Valproate use by women and girls. Medicines and Healthcare products Regulatory Agency. 2024. https://www.gov.uk/guidance/valproate-use-by-women-and-girls
- 14.Ko JM. Genetic Syndromes associated with Congenital Heart Disease. Korean Circ J. 2015;45(5):357. doi:10.4070/kcj.2015.45.5.357
- 15.Screening tests in pregnancy. NHS. 2021. https://www.nhs.uk/pregnancy/your-pregnancy-care/screening-tests/
- 16.Presentation: Clinical suspicion of Patau syndrome (trisomy 13). NHS England: Genomics Education Programme. May 2024. https://www.genomicseducation.hee.nhs.uk/genotes/in-the-clinic/presentation-clinical-suspicion-of-patau-syndrome-trisomy-13/
- 17.Presentation: Clinical suspicion of Edwards syndrome (trisomy 18). NHS England: Genomics Education Programme. April 2024. https://www.genomicseducation.hee.nhs.uk/genotes/in-the-clinic/presentation-clinical-suspicion-of-edwards-syndrome-trisomy-18/
- 18.Down syndrome (trisomy 21). NHS England: Genomics Education Programme. March 2023. https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/down-syndrome-trisomy-21/
- 19.Presentation: Clinical suspicion of 22q11.2 deletion syndrome. NHS England: Genomics Education Programme. May 2024. https://www.genomicseducation.hee.nhs.uk/genotes/in-the-clinic/clinical-suspicion-of-22q112-deletion-syndrome/
- 20.Turner syndrome. NHS England: Genomics Education Programme. March 2023. https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/turner-syndrome/
- 21.20-week screening scan. NHS England. 2024. https://www.gov.uk/government/publications/fetal-anomaly-screening-programme-handbook/20-week-screening-scan
- 22.Sun HY. Prenatal diagnosis of congenital heart defects: echocardiography. Transl Pediatr. 2021;10(8):2210-2224. doi:10.21037/tp-20-164
- 23.Personal Child Health Record (PCHR). Royal College of Paediatrics and Child Health. https://www.rcpch.ac.uk/resources/personal-child-health-record-pchr
- 24.Pushparajah K, Duong P, Mathur S, Babu-Narayan SV. Cardiovascular MRI and CT in congenital heart disease. Echo Res Pract. 2019;6(4):R121-R138. doi:10.1530/erp-19-0048
- 25.Atrial septal defect. British Heart Foundation. December 14, 2023. https://www.bhf.org.uk/informationsupport/conditions/atrial-septal-defect
- 26.Heart Conditions in Children Information Sheet: Ventricular Septal Defect. Children’s Heart Federation. May 2013. https://chfed.org.uk/wordpress/wp-content/uploads/2022/05/Ventricular-Septal-Defect.pdf
- 27.Patent ductus arteriosus. BMJ Best Practice. 2025. https://bestpractice.bmj.com/topics/en-gb/766
- 28.Coarctation of the Aorta (CoA). American Heart Association. https://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects/coarctation-of-the-aorta-coa
- 29.Newborn and infant physical examination (NIPE) screening programme handbook. NHS England. 2025. https://www.gov.uk/government/publications/newborn-and-infant-physical-examination-programme-handbook/newborn-and-infant-physical-examination-screening-programme-handbook
- 30.Lin PW, Stoll BJ. Necrotising enterocolitis. The Lancet. 2006;368(9543):1271-1283. doi:10.1016/s0140-6736(06)69525-1
- 31.Tetralogy of Fallot. BMJ Best Practice. 2024. https://bestpractice.bmj.com/topics/en-gb/701
- 32.Transposition of the great arteries. NHS England: Genomics Education Programme. March 2023. https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/transposition-of-the-great-arteries/
- 33.Atrioventricular septal defect (AVSD). British Heart Foundation. July 29, 2024. https://www.bhf.org.uk/informationsupport/conditions/atrioventricular-septal-defect-avsd
- 34.Heart Conditions in Children – Hypoplastic Left Heart Syndrome (HLHS) Information Sheet. Children’s Heart Federation. https://chfed.org.uk/heart-conditions-in-children-hypoplastic-left-heart-syndrome-hlhs-information-sheet/
- 35.British National Formulary for Children . British National Formulary for Children . https://bnfc.nice.org.uk/
- 36.Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. European Heart Journal. 2020;42(6):563-645. doi:10.1093/eurheartj/ehaa554
- 37.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary. Journal of the American College of Cardiology. 2019;73(12):1494-1563. doi:10.1016/j.jacc.2018.08.1028
- 38.Formulary 2024 (6th edition). Royal College of Paediatrics and Child Health. March 2019. https://www.rcpch.ac.uk/resources/use-unlicensed-medicines-or-licensed-medicines-unlicensed-applications-paediatric
- 39.The use of unlicensed medicines or licensed medicines for unlicensed applications in paediatric practice. Royal College of Paediatrics and Child Health. March 2019. https://www.rcpch.ac.uk/resources/use-unlicensed-medicines-or-licensed-medicines-unlicensed-applications-paediatric
- 40.Neonatal and Paediatric Pharmacists group. Position statement 2020-01 Choosing an Oral Liquid Medicine for Children. Royal College of Paediatrics and Child Health. 2020. https://nppg.org.uk/wp-content/uploads/2020/12/Position-Statement-Liquid-Choice-V1-November-2020.pdf