Zephyr / Science Photo Library
Stroke was first described more than 2,400 years ago as apoplexy, which means “struck down by violence”. As stroke is of sudden onset and often causes paralysis, this description is apt[1]
. In 1658, Jacob Wepfer studied apoplexy post mortem, and was the first to identify that apoplexy could result from either bleeding in the brain (haemorrhagic stroke) or the blockage of one of the main arteries to the brain (ischaemic stroke)[2]
.
In 2015, stroke was the second largest cause of death (after ischaemic heart disease), and accounted for 6.3 million deaths globally. Of these, ischaemic stroke was responsible for around 3 million deaths, and haemorrhagic stroke for 3.3 million deaths[3]
.
There has been a 21% reduction in the number of strokes globally[3]
; however, in the UK, even though the number of strokes has reduced from 152,000 in 2013 to 100,000 in 2015[4]
, stroke currently is the fourth largest cause of death. The 100,000 strokes that occur each year in the UK equate to one stroke occurrence every five minutes[5]
. One in eight strokes are fatal within the first 30 days[5]
, the risk of recurrent stroke is greatest within the first 30 days of having a stroke, and two-thirds of all stroke survivors will have some form of disability[5]
.
The best predictors of stroke recovery at three months are the initial neurological deficit (e.g. weakness of side of face, and/or arm, and/or leg; loss of sensations; loss of visual field; slurred speech) and age. Other factors include high blood glucose concentrations, body temperature, and previous stroke, which can result in poorer prognosis[6]
.
The estimated economic cost of stroke in the UK each year is around £9bn, with direct costs of around £4.3bn, informal care costs of around £2.4bn and loss of productivity of around £1.3bn[5]
. When looking at treatments, the initial cost of a single thrombolysis treatment is £480, with a day on a hyperacute stroke unit costing on average £583[7],[8]
. On average, the cost of care for each stroke patient is around £22,000, which includes acute care and rehabilitation[9]
.
In light of the many pressures currently facing the NHS, as well as an ever increasing ageing population, pharmacists and healthcare professionals should be aware of the risk factors for stroke, its classification and diagnosis, as outlined in this article.
Stroke types and causes
Strokes can be classified into two main types: ischaemic (i.e. caused by a clot in a blood vessel in the brain), or haemorrhagic (i.e. caused by a bleed in the brain)[5]
. These two main classifications allow further classification into subtypes (see Figure 1).
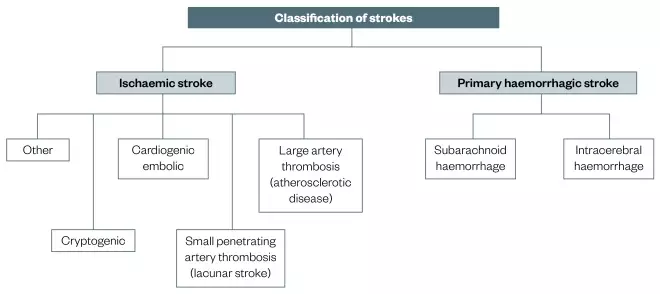
Figure 1: Classification of strokes
Strokes can be divided into ischaemic and primary haemorrhagic strokes, which can then be divided into further subcategories. Haemorrgahic stroke accounts for 15% of all strokes.
Ischaemic stroke is defined as an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction with symptoms persisting for more than 24 hours, whereas a transient ischaemic attack (TIA) is defined as a “transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischaemia without acute infarction”[10],[11]
. TIAs are typically referred to as mini-strokes with symptoms being transient (i.e. lasting from minutes to hours but less than 24 hours)[5]
.
There are two different types of haemorrhagic stroke: subarachnoid haemorrhage (SAH), which comprises around 5% of all strokes, and intracerebral haemorrhage (ICH), which accounts for around 10% of all strokes. SAH is the result of a haemorrhage from a cerebral blood vessel, aneurysm or vascular malformation into the subarachnoid space, the space surrounding the brain where blood vessels lie between the arachnoid and pia mater (see Figure 2).
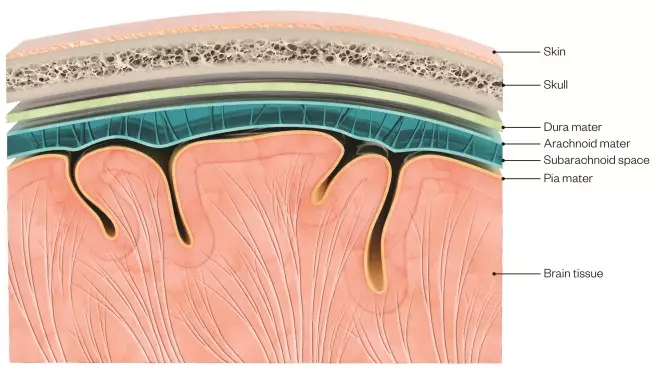
Figure 2: A brain and skull cross section showing the different anatomical layers
Source: Claus Lunau / Science Photo Library
Subarachnoid haemorrhage is the result of a haemorrhage from a cerebral blood vessel, aneurysm or vascular malformation into the subarachnoid space, the space surrounding the brain where blood vessels lie between the arachnoid and pia mater.
In SAH, patients typically experience the sudden onset of severe headache and vomiting, with non-focal neurological signs that may include loss of consciousness and neck stiffness[12]
.
Stroke caused by ICH is defined as “rapidly developing clinical signs of neurological dysfunction attributable to a focal collection of blood within the brain parenchyma or ventricular system that is not caused by trauma”[10]
.
ICH occurs spontaneously or when a weakened blood vessel within the brain bursts, allowing blood to leak, increasing intracranial pressure, causing damage to the brain cells surrounding the blood. Growth of the haematoma volume is associated with a poorer functional outcome and increased mortality rate[13]
.
To assist with the clinical diagnosis of stroke, three questions must be answered:
1. Is the process vascular or a stroke-like mimic?
2. If a vascular process, then where in the central nervous system (CNS) is the abnormality, and which blood vessels supply that area?
3. What is the disease mechanism (i.e. ischaemic or haemorrhagic)?
Clinicians should determine whether these findings could be caused by a non-vascular process (e.g. a brain tumour, metabolic disorder, infection, demyelination, intoxication or traumatic injury) that mimics stroke. Bedside clinical assessment to determine the stroke mechanism should include past and present personal and family illnesses; the presence and nature of past strokes and/or TIAs; activity at the onset of the stroke; temporal course and progression of the focal symptoms and findings; and accompanying symptoms (e.g. headache, vomiting and decreased level of consciousness)[4]
. Determination of the stroke location is most often made by assimilating all the available information, which includes the physical neurological symptoms, as well as findings from brain imaging scans.
Stroke classification: mechanisms and brain territory
Ishaemic stroke
A system for categorisation of ischaemic stroke subtypes, mainly based on aetiology and the mechanism, leading to the vessel occlusion, was developed for Trial of Org 10172 in Acute Stroke Treatment (TOAST)[14]
. This information is important in everyday management, as it should influence both the acute and secondary prevention strategies.
The TOAST classification is the most widely used and includes:
1. Large-vessel atherothrombosis;
2. Cardioembolism;
3. Small-vessel disease;
4. Other determined causes;
5. Undetermined causes.
The undetermined causes also include cases involving more than one primary mechanism[14],[15]
.
Large vessel atherothrombosis refers to the formation of lipid-laden atherosclerotic plaques on the inner wall of a large vessel and can affect both extracranial and intracranial arteries[15]
. The most common sites for formation of atherosclerotic plaques include where the common carotid arteries split, the start of the vertebral arteries and the course of the middle cerebral artery[16]
. In atheroembolism, a thrombus forms on the wall of a blood vessel, breaks apart and sheds pieces of clot, which are carried downstream and lodge in smaller arterial branches, resulting in multiple smaller strokes within the expected territory of the parent vessel[17]
.
Cardioembolism occurs as a result of blood clots, which may have formed within the heart, breaking loose, entering the circulation and then becoming lodged downstream in a cerebral artery. Clots can form within the heart because of intracardiac stasis of blood (e.g. atrial fibrillation) or as a result of adhering to a thrombogenic device or lesion (e.g. an implanted prosthetic valve)[18],[19]
.
Small-vessel disease refers to occlusive disease involving the microcirculation of the brain. Common locations for small-vessel disease include deep areas of the hemispheric white matter; the region of white matter known as the internal capsule, next to the proximal middle cerebral artery and supplied with blood by its penetrating branches; the pons in the mid-brainstem, supplied by penetrators arising from the basilar artery; and the thalamus, reliant primarily on branches of the posterior cerebral arteries[20]
. Infarctions in these regions are small (<1.5cm) and depending on the location within the brain, usually produce one of the classic lacunar syndromes[21]
:
1. Pure motor symptoms (usually face and arm, or arm and leg, comprising 33–50% of all small-vessel strokes)[22]
;
2. Pure sensory;
3. Mixed sensorimotor;
4. Ataxic hemiparesis (weakness of one side with disproportionate clumsiness [ataxia] of the same side);
5. Clumsy hand dysarthria, clumsiness of either hand, which is out of proportion to any weakness of the limb, combined with slurred speech[23]
.
Other determined causes include strokes caused by extracranial arterial dissections, non-atheroscleotic vasculopathies, hypercoagulable states or haematologic disorders[14]
.
Undetermined causes include patients in whom a complete screening workup for cardiac conduction or structural abnormalities, intracranial or extracranial large-artery stenosis, coagulopathy, and other conditions do not reveal any cause[24],[25]
. Around 40% of ischaemic strokes are of undetermined cause[26]
.The stroke may be considered to be cryptogenic after standard assessments when clinical examination and neuroimaging suggest a superficial or large, deep cerebral infarct, but none of the above routine vessel-imaging, cardiac or haematologic tests have revealed a probable cause of the stroke[27]
. Cryptogenic embolism has recently been given the term Embolic Stroke of Unknown Source (ESUS)[28]
.
The Oxfordshire Community Stroke Project (OCSP), also known as Oxfordshire or Bamford’s classification (see Box 1), relies exclusively on clinical findings to classify the stroke according to the brain territory involved and can be completed in the Accident and Emergency room[29]
. The Oxfordshire classification describes four syndromes that are either ischaemic or haemorrhagic (e.g. total anterior circulation syndrome [TACS], if ischaemic, is TACI, or if haemorrhagic, is TACH).
Box 1: The Oxfordshire Community Stroke Project (OCSP) classification[29]
This classification, also known as Oxfordshire or Bamford’s classification, relies exclusively on clinical findings. It classifies strokes according to the brain territory involved and can be completed in the Accident and Emergency room.
Total anterior circulation syndrome (TACS) includes all of:
- Unilateral motor, sensory deficit, or both affecting at least two of face, arm and leg;
- Higher cerebral dysfunction (e.g. dysphagia [swallowing disorder], dyspraxia [coordination disorder], neglect, dyscalculia [difficulty in understanding numbers];
- Homonymous hemianopia.
If consciousness is impaired, higher cerebral dysfunction and visual fields are assumed.
Partial anterior circulation syndrome (PACS):
Two of the three components of TACS or pure higher cortical dysfunction, or pure motor or sensory deficit, but not as extensive as for lacunar syndromes (see below).
Lacunar syndrome (LACS):
- Pure motor or pure sensory deficit affecting at least two of face, arm or leg;
- Sensorimotor deficit;
- Ataxic hemiparesis;
- Dysarthria (slurred speech), clumsy hand syndrome;
- Acute onset movement disorder.
Posterior circulation syndrome (POCS):
- Isolated heminopia;
- Brain stem signs;
- Cerebellar ataxia (inability to coordinate balance, gait, extremity and eye movements).
One-year mortality in the OCSP classification for the 60–70% of patients with TACS is higher than for those patients with partial anterior circulation syndrome (PACS) and posterior circulation syndrome (POCS; around 15–23%), which, in turn, is higher than for patients with lacunar syndrome (LACS; 10–15%)[30],[31]
.
Risk factors
Risk factors for stroke are classified as modifiable or non-modifiable (see Table 1)[32],[33]
. Common modifiable risk factors that are less specific and more prevalent include hypertension, diabetes and smoking, which all affect health in several ways and provide opportunities to modify risk in large numbers of people. Other specific risk factors that are less prevalent include atrial fibrillation and TIAs[34]
. Non-modifiable risk factors include age (stroke risk doubling with each decade of life after the age of 55 years), gender (more men have strokes than women; however, more women die of strokes) and genetic factors (e.g. Fabry’s disease)[35]
.
| Ishaemic | Haemorrhagic | |
|---|---|---|
| Modifiable factors | Cardiac disease | Anticoagulation |
| History of hypertension | Hypertension | |
| Diabetes | Heavy drinking | |
| Hypercholesterolaemia | Illegal drug use (especially cocaine and crystal meth) | |
| Transient ishaemic attack | Thrombolytic therapy | |
| Cigarette smoking | ||
| Hyperhomocysteinemia | ||
| Mitral stenosis | ||
| Obesity and low physical activity | ||
| Non-modifiable factors | Age | Age |
| Gender | Race/ethnicity | |
| Hereditary/familial factors | Amyloid angiopathy | |
| Race/ethnicity | ||
| Geographic location |
Haemorrhagic stroke
The primary pathology of haemorrhagic stroke is an area of bleeding that directly causes damage to the brain tissue. A third of patients presenting with ICH will, within the first few hours, have rapidly expanding haematomas. This, together with age and neurological deficit, is predictive of poor outcome at three months[36]
.
The leaking blood results in the displacement and compression of nearby tissue, which eventually dissects into ventricles and subarachnoid space. Patients may also present with additional symptoms of severe headache (due to increased intracranial pressure or meningeal irritation), lower Glasgow Coma Scale (GCS), vomiting, neck stiffness and coma.
Hypertension is the most powerful modifiable risk factor for ICH. A significant proportion of hypertensive haemorrhages occur due to non-adherence of antihypertensive medication, which can be intentional or unintentional, or as a result of illicit catecholaminergic drug use (e.g. cocaine or methamphetamine)[37]
.
A large proportion of patients with ICH are taking anticoagulants, usually prescribed for primary or secondary prevention of cardioembolic stroke in the setting of valvular heart disease or atrial fibrillation, or for the prevention or treatment of deep vein thrombosis or pulmonary embolism. Anticoagulant-related ICH accounts for up to 19% of all ICH cases and antithrombotic-associated-ICH comprises 12–20% of patients with ICH[38],[39],[40],[41]
.
The risk of inÂtracerebral bleeding is reduced in patients with atrial fibrillation who are treated with direct oral anticoagulants (DOACs) compared with patients treated with warfarin[42]
.
Haemorrhagic conversion of ischaemic stroke
This may occur after thrombolytic drugs (alteplase) are administered to patients with an ischaemic stroke or where a patient has a large cerebral clot, which tends to be more common with cardioembolic strokes[43]
. The major adverse effect of thrombolysis is symptomatic intracerebral haemorrhage, observed in around 6–7% of cases. The risk of symptomatic ICH increases with age, high blood pressure, very severe neurological deficits, severe hyperglycaemia, and possibly, with early ischaemic changes on computed tomography (CT) scans[44],[45]
.
Stroke screening tests
FAST
In February 2009, the Department of Health launched the “Act FAST” campaign to raise awareness about the signs of stroke and encourage people to call 999 immediately, so that those experiencing a stroke can be treated within 4.5 hours of onset, when they have a greater chance of receiving thrombolysis[46],[47]
.
FAST is a simple mnemonic that anyone can use and helps to identify the key symptoms of stroke. These are:
- F acial weakness
- Can the person smile?
- Has their mouth or eye drooped?
- A rm weakness
- Can the person raise both arms?
- S peech problems
- Can the person speak clearly and understand what you say?
- T ime
- Time to call 999.
Even if a person only has one of these symptoms, they or a bystander should call for an ambulance as soon as possible. As part of the patient assessment, ambulance crews monitor the patient’s blood pressure and blood sugar, and conduct an electrocardiogram (ECG). The FAST campaign led to a fall in the delay to seek and receive medical attention after a major stroke in the UK. Patients who were FAST+ were taken to hospital quicker and if a stroke was confirmed, patients have been treated more effectively[48],[49]
.
ROSIER scale
The ‘Recognition of stroke in emergency room’ (ROSIER) scale is designed for use in emergency departments[50]
. It is more in-depth than the FAST test and includes screening for common conditions that can mimic stroke, such as hypoglycaemia. Points are allocated based on presenting symptoms (see Table 2).
| Symptoms | Points |
|---|---|
| Loss of consciousness or syncope | -1 |
| Seizure activity | -1 |
| New acute onset: | |
| Asymmetric facial weakness | +1 |
| Asymmetric arm weakness | +1 |
| Asymmetric leg weakness | +1 |
| Speech disturbance | +1 |
| Visual field defect | +1 |
A stroke is likely if a person scores >0 in the absence of hypoglycaemia.
The ROSIER scale can be completed in less than five minutes; however, it is not used very often, since every minute counts, especially if a patient is a candidate for thrombolysis.
Time is brain
The human brain is densely packed with a vast network of neurons, synapses and nerve fibres. There are around 130 billion neurons in human brain; however, for every minute that a patient with an ischaemic stroke is untreated, 1.9 million neurons, 14 billion synapses and 12km (7.5 miles) of myelinated fibres are destroyed. The phrase “time is brain” is apt because strokes are medical emergencies that require urgent treatment[51]
. Prompt treatment with thrombolysis within 4.5 hours significantly improved clinical outcomes in patients with acute ischaemic stroke by reducing further neuronal destruction[48]
.
Computed tomography versus magnetic resonance imaging scans
Clinically, it is impossible to reliably distinguish cerebral infarction from intracerebral haemorrhage; this can be achieved only with non-contrast CT or magnetic resonance imaging (MRI) of the brain.
After obtaining a clear history of the onset of the symptoms and the clinical examination of the patient, the suspected stroke patient is sent for brain imaging. A CT scan can quickly exclude the presence of haemorrhage and in potential thrombolysis candidates, contribute towards decreasing the door-to-needle time for thrombolysis. A delay in CT scanning results in a longer door-to-needle time, which adversely affects a patient because of greater loss of brain neurons.
The absence of haemorrhage on the CT scan supports the diagnosis of an ischaemic event, and some evidence of ischaemia may be observed on CT; however, the diagnosis of an ischaemic stroke is based on the clinical examination, as a clot is not always observed on a CT scan.
CT scans are faster, more readily available, less expenÂsive than MRI scans, and can be performed on patients with implanted devices (e.g. pacemakers) and on patients who are claustroÂphobic.
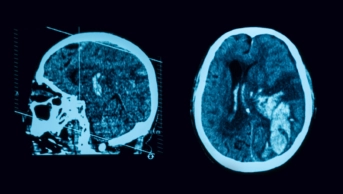
On an uncoloured CT scan a clot shows as a hyperdense sign (i.e. will look whiter than normal tissue), reflecting the arterial territory involved. Figure 3 shows a coloured CT scan where the blood vessels are shown white and the clot is shown dark in the circle. The hyperdense vessel sign and signs related to the loss of contrast between the grey and white matter (e.g. the insular ribbon sign and lentiform obscuration) are all examples of signs of acute ischaemia on CT[52]
.
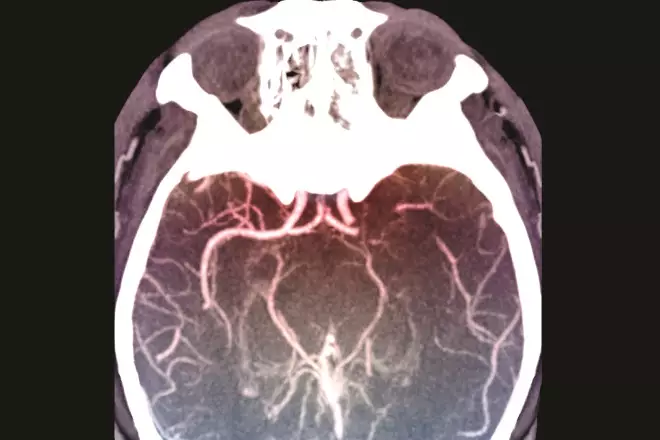
Figure 3: Middle cerebral artery clot on computed tomography (CT) scan
Source: Zephyr / Science Photo Library
Coloured computed tomography (CT) scan of an axial section through the brain of a 53-year-old patient affected by hemiplegia (paralysis) of the right side of the body following a stroke. A thrombosis is visible at the termination of the left internal carotid artery.
Neuroimaging also serves to exclude other pathologies that may resemble stroke clinically. These stroke mimics include, haemorrhagic neoplasms, encephalitis, multiple sclerosis, postictal (Todd’s) paresis, some types of migraine, intoxications, hypertensive encephalopathy, and hyper- or hypo-glycaemia[52]
.
An acute haemorrhage on CT is clearly visible in the acute phase, as high attenuation. This appearance remains reliable for around 72 hours. By day 10, the haemorrhage becomes hypodense and becomes indistinguishable from an infarct[53],[54]
.
Multimodal MRI sequences, particularly diffusion-weighted images, depict anatomy in greater detail than non-conÂtrast CT; however, MRI is more time-consuming than CT scanning and is, therefore, not appropriate for the initial assessment of patients who may be eligible for thrombolysis. MRI is more sensitive in detecting early ischaemia and it allows for differentiation between old and new ischaemia[55]
. MRI is preferred for the investigation of TIA[56]
.
The major drawback of CT is the high radiation dose, while with MRI it is more complicated and time consuming[52]
.
Role of the pharmacy team on the acute stroke unit
The pharmacy team, which includes specialist stroke pharmacists and pharmacy technicians, plays an integral role in the multidisciplinary stroke team (MDT) in assessing the stroke patient’s physical and cognitive disabilities, and in managing their medication. Knowing what type of stroke a patient has, whether it is total anterior circulation syndrome (TACS), partial anterior circulation syndrome (PACS), posterior circulation syndrome (POCS) or lacunar syndrome (LACS), and the MDT’s description of the patient’s symptoms and deficits, can assist the pharmacist in assessing the patient’s disability and medication challenges (e.g. dysphagia, aphasia, administration and adherence).
For example, if a patient has TACS, then they will have the following symptoms:
- Unilateral weakness and/or sensory deficit of face, arm and leg;
- Homonymous hemianopia (see Figure 4);
- Higher cerebral dysfunction like dysphasia, visuospatial disorder.
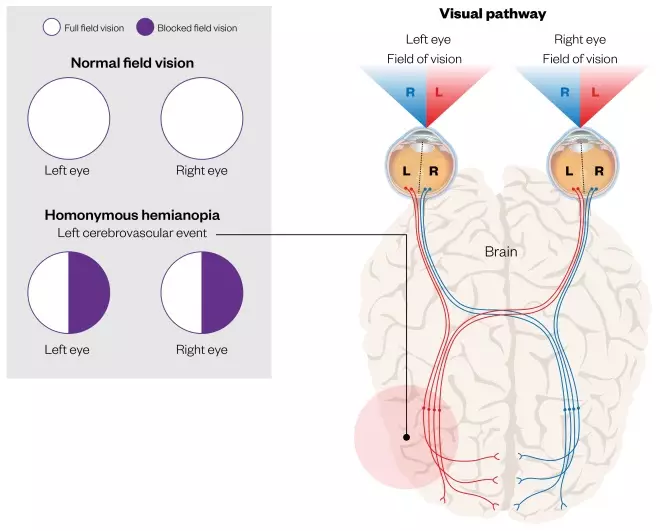
Figure 4: Homonymous hemianopia as a result of a cerebrovascular event
Source: Shutterstock.com / MAG
Homonymous hemianopia is hemianopic visual field loss on the same side of both eyes. Homonymous hemianopia occurs because the right half of the brain has visual pathways for the left hemifield of both eyes, and the left half of the brain has visual pathways for the right hemifield of both eyes. When one of these pathways is damaged, the corresponding visual field is lost.
The degree of the patient’s stroke symptoms is linked to the location and size of the infarct/haemorrhage.
If the patient has a left-sided homonymous hemianopia, it would be advisable for pharmacists and healthcare professionals, when communicating with the patient, to stand on the patient’s right side. This would allow for the patient to see them and allow for an enhanced patient consultation. Speech and language therapists (SLTs) are able to guide healthcare professionals on using appropriate techniques and tools when communicating with aphasic patients. SLTs are able to assess the patient for dysphagia and recommend fluid consistency and food texture to allow for safe swallowing. Using this information, pharmacists can amend oral medication formulations to allow for the safe and appropriate administration of oral medication where necessary[57]
.
With upper limb hemiparesis, assessing the strength and tone of the patient’s arm and hand will provide evidence as to whether the patient would manage administering oral medication to themselves, taking into account any cognitive impairment post-stroke.
Financial and conflicts of interest disclosure:
The author has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Thompson JE. The evolution of surgery for the treatment and prevention of stroke. The Willis Lecture. Stroke 1996;27(8):1427– 1434. doi: 10.1161/01.STR.27.8.1427
[2] Fields WS & Lemak NA. A History of Stroke. New York, NY: Oxford University Press; 1989.
[3] GBD 2015 Mortality and Causes of Death, Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1459–1544. doi: 10.1016/s0140-6736(16)31012-1
[4] Royal College of Physicians Sentinel Stroke National Audit Programme (SSNAP). Is stroke care improving? Second SSNAP Annual Report prepared on behalf of the Intercollegiate Stroke Working Party November 2015. Available at: https://www.strokeaudit.org/getattachment/AnnualReport/Historical-Guideline/Apr2014Mar2015-AnnualReport.pdf.aspx (accessed January 2018)
[5] Stroke Association (2017). State of the Nation — Stroke statistics [online]. Available at: https://www.stroke.org.uk/resources/state-nation-stroke-statistics (accessed January 2018)
[6] Weimar C, Ziegler A, Konig IR et al. Predicting functional outcome and survival after acute ischemic stroke. J Neurol 2002;249:888–895. doi: 10.1007/s00415-002-0755-8
[7] National Institute for Health and Care Excellence. Quality Standards Programme. NICE cost impact and commissioning assessment: quality standard for stroke. Available at: https://www.nice.org.uk/guidance/qs2/documents/stroke-quality-standards-update-qs-topic-overview2 (accessed January 2018)
[8] National Audit Office. Progress in improving stroke care. Report on the findings from our modelling of stroke care provision (February 2010). NAO Report (HC 291 2009-2010). Available at: https://www.nao.org.uk/wp-content/uploads/2010/02/0910291_modelling.pdf (accessed January 2018)
[9] Royal College of Physicians Sentinel Stroke National Audit Programme (SSNAP). Stroke health economics: cost and cost-effectiveness analysis 2016.
[10] Sacco R, Kasner S, Broderick J et al. An updated definition of stroke for the 21st Century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca
[11] Easton JD, Saver JL, Albers GW et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218
[12] van Gijn J & Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249–278. PMID: 11157554
[13] Elliott J & Smith M. The acute management of intracerebral hemorrhage: a clinical review. Anesth Analg 2010;110(5):1419–1427. doi: 10.1213/ANE.0b013e3181d568c8
[14] Adams HP Jr, Bendixen BH, Kappelle LJ et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41. doi: 10.1161/01.STR.24.1.35
[15] Rovira A, Grivé E, Rovira A & Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol 2005;15(3):416–426. doi: 10.1007/s00330-004-2633-5
[16] Derdeyn CP. Mechanisms of ischemic stroke secondary to large artery atherosclerotic disease. Neuroimaging Clin N Am 2007;17(3):303–311. doi: 10.1016/j.nic.2007.03.001
[17] Rapp JH, Pan XM, Yu B et al. Cerebral ischemia and infarction from atheroemboli <100µm in size. Stroke 2003;34(8):1976–1980. doi: 10.1161/01.STR.0000083400.80296.38
[18] Olsson SB & Halperin JL. Prevention of stroke in patients with atrial fibrillation. Semin Vasc Med 2005;5(3):285–292. doi: 10.1055/s-2005-916168
[19] Verheugt FW. Anticoagulation after artificial valve replacement with or without atrial fibrillation: how much is really needed? Thromb Haemost 1999;82(Suppl 1):130–135.
[20] Nah HW, Kang DW, Kwon SU & Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: analysis of indicators for small vessel disease and atherosclerosis. Stroke 2010;41(12):2822–2827. doi: 10.1161/STROKEAHA.110.599464
[21] Koch S, McClendon MS & Bhatia R. Imaging evolution of acute lacunar infarction: leukoaraiosis or lacune? Neurology 2011;77(11):1091–1095. doi: 10.1212/WNL.0b013e31822e1470
[22] De Reuck J, De Groote L & Van Maele G. The classic lacunar syndromes: clinical and neuroimaging correlates. Eur J Neurol 2008;15(7):681–684. doi: 10.1111/j.1468-1331.2008.02147.x
[23] Arboix A & Marti Vilalta JL. Lacunar stroke. Expert Rev Neurother 2009;9(2):179–196. doi: 10.1586/14737175.9.2.179
[24] Amarenco P. Underlying pathology of stroke of unknown cause (cryptogenic stroke). Cerebrovasc Dis 2009;27(Suppl 1):97–103. doi: 10.1159/000200446
[25] Marnane M, Duggan CA, Sheehan OC et al. Stroke subtype classification to mechanismË—specific and undetermined categories by TOAST, ASCO, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke 2010;41:1584. doi: 10.1161/STROKEAHA.109.575373
[26] Wolf ME, Sauer T, Alonso A & Hennerici MG. Comparison of the new ASCO classification with the TOAST classification in a population with acute ischemic stroke. J Neurol 2012;259(7):1284–1289. doi: 10.1007/s00415-011-6325-1
[27] Saver J. Cryptogenic Stroke. N Engl J Med 2016;374:2065–2074. doi: 10.1056/NEJMcp1503946
[28] Hart RG, Diener HC, Coutts SB et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7
[29] Bamford JM. The role of the clinical examination in the subclassification of stroke. Cerebrovasc Dis 2000;10(suppl 4):2–4. doi: 10.1159/000047582
[30] Myint PK, Bachmann M, Loke YK et al. Important factors in predicting mortality outcome from stroke: findings from the Anglia Stroke Clinical Network Evaluation Study. Age and Ageing 2017;46(1):83–90. doi: 10.1093/ageing/afw175
[31] Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol 2003;2:238–245. doi: 10.1016/S1474-4422(03)00352-1
[32] Sacco RL, Benjamin EJ, Broderick JP et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke 1997;28(7):1507–1517. doi: 10.1161/01.STR.28.7.1507
[33] Allen C & Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke 2008;3(2):105–116. doi: 10.1111/j.1747-4949.2008.00187.x
[34] Whisnant JP. Modeling of risk factors for ischemic stroke. The Willis lecture. Stroke 1997;28:1840–1844. doi: 10.1161/01.STR.28.9.1840
[35] Wolf PA, D’Agostino RB, O’Neal MA et al. Secular trends in stroke incidence and mortality: the Framingham Study. Stroke 1992;23:1551–1555. doi: 10.1161/01.STR.23.11.1551
[36] Davis SM, Broderick J, Hennerici M et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99
[37] Broderick JP. Intracerebral hemorrhage. In: Gorelick PB, Alter M, eds. Handbook of Neuroepidemiology. New York, NY: Marcel Dekker Inc; 1994.
[38] Le Roux P, Pollack C Jr, Milan M & Schaefer A. Race against the clock: overcoming challenges in the management of anticoagulant-associated intracerebral hemorrhage. J Neurosurg 2014;121(Suppl):1–20. PMID:25081496
[39] Cucchiara B, Messe S, Sansing L et al. CHANT Investigators. Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke 2008;39(11):2993–2996. doi: 10.1161/STROKEAHA.108.520668
[40] Fang MC, Go AS, Chang Y et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007;120:700–705. doi: 10.1016/j.amjmed.2006.07.034
[41] Rosand J, Eckman MH, Knudsen K et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. doi: 10.1001/archinte.164.8.880
[42] Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0
[43] Molina CA, Montaner J, Abilleira S et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 2001;32(5):1079–1084. doi: 10.1161/01.STR.32.5.1079
[44] Larrue V, von Kummer R, del Zoppo G et al. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–960. doi: 10.1161/01.STR.28.5.957
[45] Fiorelli M, Bastianello S, von Kummer R et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–2284. doi: 10.1161/01.STR.30.11.2280
[46] Department of Health (2009) Stroke: Act F.A.S.T. awareness campaign. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_094239 (accessed January 2018)
[47] Hacke W, Kaste M, Bluhmki E et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656
[48] NHS Networks 2014. Latest figures show that the annual “act fast” stroke campaign has had a significant impact on patients receiving stroke treatment. Available at: http://www.networks.nhs.uk/news/government-claims-success-for-2018act-fast2019-stroke-campaign (accessed January 2018)
[49] Flynn D, Ford GA, Rodgers H et al . A time series evaluation of the FAST national stroke awareness campaign in England. PLoS ONE 2014;9(8):e104289. doi: 10.1371/journal.pone.0104289
[50] Nor AM, Davis J, Sen B et al. The recognition of stroke in the emergency room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol 2005;4:727–734. doi: 10.1016/S1474-4422(05)70201-5
[51] Saver J. Time is brain—quantified. Stroke 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab
[52] Vymazal J, Rulseh AM, Keller J & Janouskova L. Comparison of CT and MR imaging in ischemic stroke. Insights into Imaging 2012;3(6):619–627. doi: 10.1007/s13244-012-0185-9
[53] Jauch EC, Saver JL, Adams HP Jr et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on PeriphÂeral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a
[54] Wintermark M, Sanelli PC, Albers GW et al. Imaging recommendations for acute stroke and transient ischemic attack patients: a joint stateÂment by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol 2013;34(11):E117–E127. doi: 10.1016/j.jacr.2013.06.019
[55] Nour M & Liebeskind D. Brain imaging in stroke: insight beyond diagnosis. Neurotherapeutics 2011;8:330–339. doi: 10.1007/s13311-011-0046-0
[56] National Institute for Health and Care Excellence. Diagnosis and initial management of acute stroke and transient ischaemic attack. July 2008. Available at: http://www.nice.org.uk/cg68 (accessed January 2018)
[57] Barnett N & Parmar P. How to tailor medication formulations for patients with dysphagia. Pharm J 2016;297(7892):online. doi: 10.1211/PJ.2016.20201498