Shutterstock.com
In this article you will learn:
- The causes of subfertility
- How subfertility is diagnosed
- The pharmacological and surgical options available and when they are considered
Subfertility is defined by the failure of a heterosexual couple to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse[1]
.
Primary subfertility relates to a couple who have never managed to conceive, while secondary subfertility refers to a couple who have managed to conceive (although not necessarily resulting in a live birth). At least a quarter of couples experience a period of inability to conceive lasting over one year at some point in their lives[2]
. Some of these couples continue to be unable to conceive, leading to at least one in six (17%) couples seeing a fertility specialist at a hospital in the UK[3]
.
Women become less fertile as they get older, and age is associated with a gradual decrease in both the quantity and quality of oocytes. Around 95% of women aged 35 years who have regular sexual intercourse without contraception will get pregnant after three years of trying, compared with 75% of women aged 38 years. The effect of age upon men’s fertility is less clear. Demographic data on UK fertility rates suggest that male subfertility is increasing[4]
.
This article will focus on the treatment of subfertility in women.
Physiology
Ovulation involves a progression of cellular changes in follicles in the ovary that occur from foetal life until menopause[5]
.
Following menarche, women experience a menstrual cycle that lasts, on average, 28 days. Each cycle starts with shedding of the endometrium, resulting in bleeding that typically lasts three to six days. Simultaneously, a cohort of 10–15 follicles starts to grow in the ovaries driven by the gonadotrophin follicle stimulating hormone (FSH). FSH, along with lutenising hormone (LH), which is responsible for ovulation, are released from the anterior pituitary gland when stimulated by gonadotrophin-releasing hormone (GnRH), which is secreted from the hypothalamus (see Hypothalamic-pituitary-ovarian axis)[6]
. These pathways are regulated by activin and inhibin, released from the ovaries, and estradiol (an oestrogen) and progesterone, which are produced as the dominant follicle develops by around day six to nine[7]
. This dominant follicle ovulates at around day 14; the remaining follicles undergo atresia.
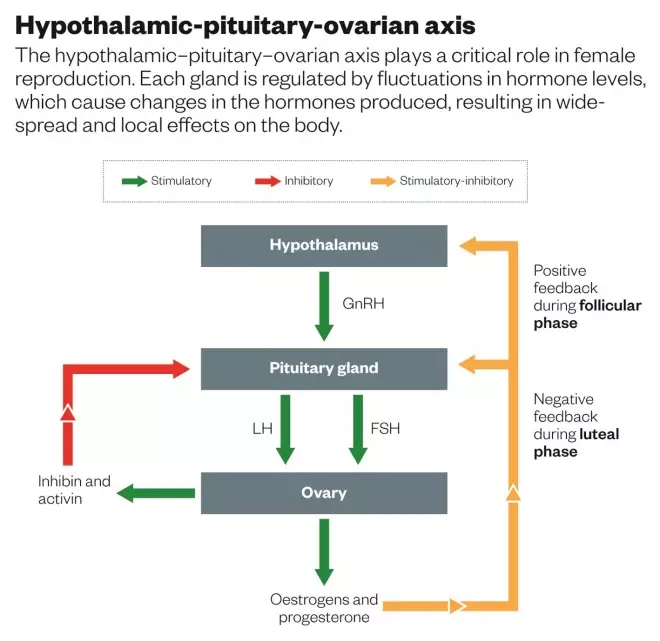
Hypothalamic-pituitary-ovarian axis
The hypothalamic–pituitary–ovarian axis plays a critical role in female reproduction. Each gland is regulated by fluctuations in hormone levels, which cause changes in the hormones produced, resulting in widespread and local effects on the body.
Causes of female subfertility
Anovulation is the failure of the ovary to release ova over a period of at least 45 days)[8]
. The World Health Organization classifies ovulatory disorders into three groups
[1]
:
- Group I: hypothalamic pituitary failure (hypothalamic amenorrhoea or hypogonadotrophic hypogonadism);
- Group II: hypothalamic-pituitary-ovarian dysfunction, predominantly polycystic ovary syndrome (PCOS);
- Group III: ovarian failure.
Polycystic ovary syndrome accounts for at least 80% of all cases of anovulation . According to the Rotterdam criteria[9]
(European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine 2003), PCOS is diagnosed if the patient has any two of the following three features: polycystic ovaries on ultrasound (≥12 antral follicles in one ovary or ovarian volume ≥10cm3); clinical hyperandrogenism (Ferriman-Gallwey Score ≥8) or biochemical hyperandrogenism (elevated total or free testosterone); and oligomenorrhoea (less than six to nine menses per year) or oligo-ovulation.
Hypogonadotropic hypogonadism, caused by impaired secretion of gonadotrophins from the pituitary gland, constitutes 5–10% of subfertility cases[8]
. This can be caused by damage to the pituitary gland or hypothalamus from surgery, injury, tumours, infections, ischemia or radiation, genetic defects (e.g. Kallmann syndrome), high doses or long-term use of opioid or steroid (glucocorticoid) medications, severe stress and chronic medical diseases, including chronic inflammation or infections and eating disorders (anorexia nervosa).
Hyperprolactinaemia, or raised prolactin levels (above 100–510mIU/L), can inhibit normal GnRH secretion from the hypothalamus, resulting in anovulation and amenorrhoea. Common causes of hyperprolactinaemia include prolactinomas (pituitary tumours), drugs (antipsychotics, antidepressants, opioids, anti-hypertensives) and hypothyroidism.
Hypergonadotropic hypogonadism causes around 3–5% cases of subfertility[8]
. This is caused by ovarian failure, and can be congenital or acquired. Congenital causes of primary amenorrhoea include chromosomal abnormalities (e.g. Turner syndrome, Klinefelter syndrome), defects in hormone synthesis and gonadotrophin resistance. Acquired causes (resulting from damage to, or dysfunction of the gonads) include premature ovarian failure or insufficiency secondary to genetic conditions (e.g. fragile X syndrome), gonadal torsion, surgery, autoimmunity, chemotherapy, radiation, infections (e.g. sexually transmitted infections or mumps), trauma and ovarian resistance syndrome.
Damage to the fallopian tubes can keep sperm from getting to the ovum, or block the passage of the fertilised ovum into the uterus. The most common cause of fallopian tube damage or blockage is pelvic inflammatory disease (often caused by sexually transmitted infections like chlamydia and gonorrhoea)[10]
.
Other causes include previous tubal (tubal ligation or recanalisation) surgeries, abdominal or pelvic surgery, pelvic tuberculosis, endometritis, endometriosis and, rarely, congenital defects (absence, duplication, diverticula) and salpingitis isthmica nodosa (nodular scarring of the fallopian tubes).
Uterine abnormalities such as fibroids, polyps, adhesions and anomalies interfere with fertility by affecting sperm migration and embryo implantation[11]
.
Uterine fibroids are common, benign tumours of the uterus that occur in up to 77% of women approaching the age of 50 years[12]
. Submucosal fibroids are associated with a 70% reduction in live birth rate compared with unaffected women, while intramural fibroids not distorting the endometrial cavity reduce live birth rates by 21% compared with unaffected women[13]
. Subserosal fibroids are not associated with a reduction in fertility.
Little is known about the association between endometrial polyps and subfertility. However, despite this lack of evidence, most clinicians recommend hysteroscopic removal, and conception rates in subfertile women are between 35% and 84% following surgery[14]
.
Uterine anomalies such as septums can also reduce fertility and are usually treated with surgery[15]
.
Endometriosis affects 20–40% of women with history of subfertility, compared with a background prevalence of 5–10% in the general female population[16]
. Possible reasons for subfertility associated with endometriosis include painful intercourse; alterations in pelvic anatomy or the peritoneal and tubal environment; or interference with fertilisation, embryo development and implantation[17]
.
The immune system is involved in all aspects of reproduction, particularly around conception. Immune parameters at this early phase are pivotal for shaping the viability of a pregnancy, the growth of the foetus, and offspring health after birth. It is now clear that immune cells are positioned and equipped to respond to detect antigens and other signals originating in seminal fluid, the embryo and placental trophoblasts. Suppressing maternal immunity with the use of immune therapies, such as immunoglobulin infusions, corticosteroids or Intralipid (which acts to lower the activity of NK cells, allowing the embryo to implant on the uterine wall and grow normally), has been shown to increase live birth rates in women displaying abnormal immunological risk factors[18]
.
Lifestyle factors can affect fertility. Women with a body mass index (BMI) <19 may have irregular menstrual cycles, which can decrease their chance of conception. Women and men who have a BMI >30 take longer to conceive. Obesity mainly interferes with ovulation in women and sperm quality in men.
Smoking and recreational drugs also affect egg and sperm quality and reduce the chance of conception[19]
.
Changes to the consistency or viscosity, or the presence of antibodies, in cervical mucus has not been shown to cause subfertility.
Diagnosis
The diagnosis of the cause of subfertility is based on the couple’s history, together with the appropriate investigations. It is important for any clinician to see the couple together for any fertility discussion. Typically the first consultation will be with a GP, who will then refer the couple to a fertility specialist in secondary or tertiary care.
A patient’s history should include age of menarche, details of menstrual cycles (duration, length, frequency, associated symptoms) and previous obstetric events (miscarriages, births, ectopic pregnancies, terminations), as well as a sexual, social, psychological, medical (including drugs, contraceptives and vaccinations) and surgical history.
Patients will receive a physical examination, involving a determination of BMI and a general examination (to rule out hirsutism, acne, enlargement of thyroid and galactorrhoea), before including assessment of secondary sexual characteristics with abdominal palpation. A speculum will be used to view the woman’s vagina and cervix, and a bimanual pelvic examination will be performed to assess uterine, tubal or ovarian pathology.
Common baseline investigations for subfertility in women include:
- Mid-luteal phase (day 21 of a 28 day cycle) progesterone (ovulation >30nmol/L, anovulation <30nmol/L);
- Early follicular phase (day 1–4) hormone levels: FSH (normal level 4–9 IU/L, menopausal >30 IU/L), LH (normal level 4–9 IU/L, menopausal >30 IU/L) and estradiol (normal < 200pmol/L);
- Anti-Müllerian hormone (normal fertility 6.9–18pmol/L);
- Rubella immunity (this is to ensure the foetus is protected; rubella does not affect fertility);
- Genital swabs for infection screening (e.g. chlamydia, gonorrhoea);
- Cervical smear;
- Tube patency testing;
- Hormone profile, including testosterone, sex-hormone binding globulin (SHBG), free androgen index (FAI), thyroid-stimulating hormone (TSH), free thyroxine, prolactin;
- Transvaginal scan to investigate the uterus, endometrium, ovaries and fallopian tubes.
A diagnosis of unexplained subfertility is made if investigations fail to reveal any abnormality.
Treatment in anovulatory women
The choice of management depends on the woman’s age, cause and duration of subfertility, and the woman’s preference. Evaluation and treatment should begin earlier if the woman is aged 35 years or older.
Medical treatment in anovulatory women aims to stimulate monofollicular development up to the preovulatory stage that results in ovulation. Medicines used for ovulation induction are associated with risks of multiple pregnancies and ovarian hyperstimulation syndrome (OHSS). Although it has been suggested that using medicines to stimulate ovaries may increase the risk of ovarian cancer, this is unconfirmed and the risk is considered to be very low.
Group I anovulatory subfertility is treated by replacing the deficient gonadotrophins FSH and LH
[20]
. They are available in recombinant and highly purified urinary forms (menopausal gonadotrophins). They are usually given in the dose from 37.5–75IU, and a transvaginal scan is performed to track ovulation response. Once a follicle reaches the size of 18mm, ovulation can then be achieved naturally or triggered by a 5,000–10,000IU human chorionic gonadotrophin (hCG) injection.
Women with hyperprolactinemia can be treated with an oral dopamine agonist (e.g. bromocriptine, cabergoline), which will induce normo-prolactinemia and ovulatory cycles[21]
.
GnRH analogues (e.g. leuprorelin, nafarelin, goserelin) can be used to activate the GnRH receptor on the pituitary gland, resulting in increased secretion of FSH and LH. Initially, it was thought they could be used as potent and prolonged stimulators of gonadotrophins from the pituitary gland; however, after an initial ‘flare’ effect they eventually cause a sustained reduction in gonadotrophin secretion after about ten days. This ‘downregulation’ phase is reversed on stopping treatment.
Group II constitutes 80% of all cases of anovulation[4]
. The treatment of choice is clomifene citrate 50–150mg, given for five days starting on cycle day 3, 4 or 5. Clomifene is a selective oestrogen receptor modulator that inhibits oestrogen receptors in the hypothalamus, thus inhibiting negative feedback of oestrogen on gonadotrophin release. Successful ovulation is achieved in 70–85% of women who receive treatment, and around 40–50% will conceive. If clomifene is unsuccessful in achieving ovulation, gonadotrophins can be used[22]
.
Metformin (500–1,500mg) can also be used alone or in combination with clomifene to induce ovulation. It may be successful in certain subgroups such as obese women or women with hyperandrogenism.
Group III patients require egg donation treatment as ovulation cannot be induced in these patients.
Surgical treatment
Women with uterine abnormalities that may affect fertility may be offered surgery, depending on the cause of subfertility.
Uterine surgery may be indicated for submucosal and intramural fibroids, which are more likely to interfere with conception than subserosal fibroids. Surgical removal of fibroid (myomectomy) is recommended to improve the chances of conception and reduce miscarriage rate, occurring naturally as well as with fertility treatments.
Hysteroscopic resection may be performed in women with a uterine septum or adhesion, which can improve conception and reduces miscarriage rates. The underlying mechanisms interfere with fertility by affecting sperm migration and embryo implantation.
Endometrial surgery may be performed to remove endometrial polyps or adhesions to improve conception rates naturally and prior to in vitro fertilisation (IVF).
Laparoscopic ovarian drilling is an option for patients in whom clomifene is not effective in inducing ovulation.
Peritoneal surgery may be considered to treat superficial endometriosis, and is associated with increased spontaneous conception rates.
Assisted reproduction
Intrauterine insemination (IUI) involves a laboratory procedure where fast-moving sperm are placed into the woman’s womb close to the time of ovulation, when the egg is released from the ovary, usually on day 14 of the monthly cycle. It is indicated in unexplained subfertility, psychosexual problems, pain during intercourse or when sperm donation is used. It can also be used in cases of male impotence, or in couples where one partner is infected with the human immunodeficiency virus (HIV).
In vitro
fertilisation involves eggs being harvested from the ovaries and fertilised with sperm in a laboratory. The fertilised egg (embryo) is later placed in the woman’s womb. It is indicated in women with unexplained subfertility, blocked fallopian tubes, male factor subfertility, unsuccessful pharmacological treatment or IUI. In the UK, limited IVF treatment is usually available on the NHS if the couple meets the following criteria, although there are local variations:
- Woman is aged 21–39 years;
- Both partners are non-smokers;
- Woman’s BMI is 19–30;
- No living children from present or past relationship by either partner;
- Both partners living at the same address;
- Partners are in a stable relationship;
- Neither partner may have had a sterilisation procedure in the past.
IVF involves several steps. First, the woman’s natural hormone cycle is supressed using a GnRH agonist (e.g. buserelin, nafarelin) or GnRH antagonist (e.g. cetrorelix) to suppress endogenous release of FSH and LH. This is followed by controlled ovarian stimulation with gonadotrophins, in doses ranging from 75IU to 450IU to cause superovulation. Follicular growth is monitored by transvaginal ultrasound; once ready, patients are given an injection of hCG 36 hours before egg collection to bring about final maturation and trigger their release. Eggs are collected transvaginally under sedation or general anaesthesia by a needle inserted into each ovary, guided by transvaginal ultrasound.
Once obtained, the eggs are left with sperm to fertilise and form embryos. In cases of abnormal sperm parameters, each egg is injected with a single sperm, called intra-cytoplasmic sperm injection (ICSI). Finally, embryos are transferred to the womb. In the UK, the Human Fertilisation and Embryology Authority (HFEA) has imposed restrictions on the number of embryos that can be transferred in IVF in order to reduce the number of multiple births (e.g. twins or triplets). Women younger than 36 years are typically given one embryo, women aged 36–40 years receive up to two embryos, and women older than 40 years can receive up to three embryos.
Women receiving fertility treatment, particularly IVF, are at increased risk of OHSS, a systemic disease resulting from vasoactive products released by hyperstimulated ovaries. OHSS is characterised by increased capillary permeability, leading to leakage of fluid from the vascular compartment, with third space fluid accumulation and intravascular dehydration. Severe manifestations include thrombosis, renal and liver dysfunction and acute respiratory distress syndrome[23]
. Women suffer from mild OHSS in up to 33% of IVF cycles, and moderate or severe OHSS in 3–8% of IVF cycles[24]
.
There is an overall increased risk of ectopic pregnancy in women who have received IVF. In pregnancies with multiple gestation, there is also an increased risk of miscarriage, preterm delivery, low birth weight, hypertension, induction of labour, cerebral palsy and caesarean section[25]
.
Prashant Purohit MRCOG is a clinical research fellow, assisted conception unit, and Kate Pine is p
harmacy
team leader, women’s and children’s services, both at King’s College Hospital.
How to have effective consultations on contraception in pharmacy
What benefits do long-acting reversible contraceptives offer compared with other available methods?
Community pharmacists can use this summary of the available devices to address misconceptions & provide effective counselling.
Content supported by Bayer
References
[1] Zegers-Hochschild F, Adamson GD, de Mouzon J et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009.
[2] Gunnell DJ & Ewings P. Subfertility prevalence, needs assessment and purchasing. Journal of Public Health Medicine 1994;16:29–36.
[3] Hull MGR, Glazener CMA, Kelly NJ et al. Population study of causes, treatment and outcome of subfertility. BMJ 1985;291:1693–1697.
[4] Povey AC & Stocks SJ. Epidemiology and trends in male subfertility. Human Fertility 2010;13(4):182–188.
[5] Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrin Rev 1996;17:121–155.
[6] Meduri G, Bachelot A, Cocca MP et al. Molecular pathology of the FSH receptor: new insights in FSH pathology. Molecular and Cellular Endocrinology 2008;282:130.
[7] Veldhuis J, Rogol A, Samojlik E et al. Role of endogenous opiates in the expression of negative feedback actions of estrogen and androgen on pulsatile properties of luteinizing hormone secretion in man. J Clin Invest 1984;74:47.
[8] Farid NR & Diamanti-Kandarakis E (eds). Diagnosis and management of polycystic ovary syndrome. Springer; 2009. p243.
[9] Rotterdam criteria ESHRE-ASRM Consensus group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
[10] Ledger WL, Tan SL & Bahathiq AOS. The fallopian tube in subfertility and IVF practice. New York: Cambridge University Press; 2010. p1–130.
[11] Fatemi HM, Kasius JC, Timmermans A et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod 2010;25:1959–1965.
[12] Day Baird D, Dunson DB, Hill MC et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–107.
[13] Somigliana E, Vercellini P, Daguati R et al . Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update 2007;13:465–476.
[14] Yu D, Wong YM, Cheong Y et al. Asherman syndrome: one century later. Fertil Steril 2008;89:759–779.
[15] Woelfer B, Salim R, Banerjee S et al. Reproductive outcomes in women with congenital uterine anomalies detected by three-dimensional ultrasound screening. Obstet Gynecol 2001;98:1099–1103.
[16] Garcia-Velasco JA & Somigliana E. Management of endometriomas in women requiring IVF: to touch or not to touch. Hum Reprod 2009;24:496–501.
[17] Mahutte NG & Arici A. New advances in the understanding of endometriosis related subfertility. J Reprod Immunol 2002;55:73–83.
[18] Robertson SA & Moldenhauer LM. Immunological determinants of implantation success: Int J Dev Biol 2014;58(2–4):205–217. doi:10.1387/ijdb.140096sr.
[19] Sharma R, Biedenharn KR, Fedor JM et al. Lifestyle factors and reproductive health: taking control of your fertility: Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66.
[20] Gorthi S, Balen AH & Tang T. Current issues in ovulation induction. The Obstetrician & Gynaecologist 2012;14:188–196.
[21] Chanson P, Borson-Chazot F, Chabre O et al. Drug treatment of hyperprolactinemia. Ann Endocrinol 2007;68(2–3):113–117.
[22] The ESHRE Capri Workshop. Female infertility: treatment options for complicated cases. Hum Reprod 1997;12(6):1191–1196.
[23] Beerendonk CC, van Dop PA, Braat DD et al. Ovarian hyperstimulation syndrome: facts and fallacies. Obstet Gynecol Surv 1998;53:439–449.
[24] Royal College of Obstetricians and Gynaecologists. The management of ovarian h yperstimulation s yndrome: RCOG Green Top Guideline No.5. September 200 6.
[25] American Society for Reproductive Medicine. Patient fact sheet: complications and problems associated with multiple births. 2008.