Shutterstock.com
After reading this article, you should be able to:
- Understand the need for a final medical signatory;
- Know the objectives of the role;
- Understand what skills, qualifications, and training is required for the position;
- Find opportunities as a final medical signatory.
Promotion of pharmaceuticals and information provided to patients and members of the public is strictly regulated by the Association of the British Pharmaceutical Society (ABPI). The ABPI Code of Practice sets out specific requirements for the UK pharmaceutical industry surrounding all aspects of medicines promotion.
The Code of Practice extends beyond UK law and is administered by the self-regulatory body, the Prescription Medicines Code of Practice Authority (PMCPA), allowing the self-regulation of pharmaceutical companies. Membership to the ABPI is voluntary, but the majority of UK pharmaceutical companies are members and therefore must follow its code. Non-members can also agree to follow the code.
The need for a final medical signatory
Final medical signatories are UK-registered doctors, pharmacists or dentists (for dentistry-specific use products) employed by pharmaceutical companies who ensure the standards and requirements of the ABPI Code of Practice are met, before approving industry-produced materials or arrangements associated with a particular activity (e.g. the organisation and delivery of therapy-review services). Registration with the PMCPA and the Medicines and Healthcare Products Regulatory Agency (MHRA) is mandatory.
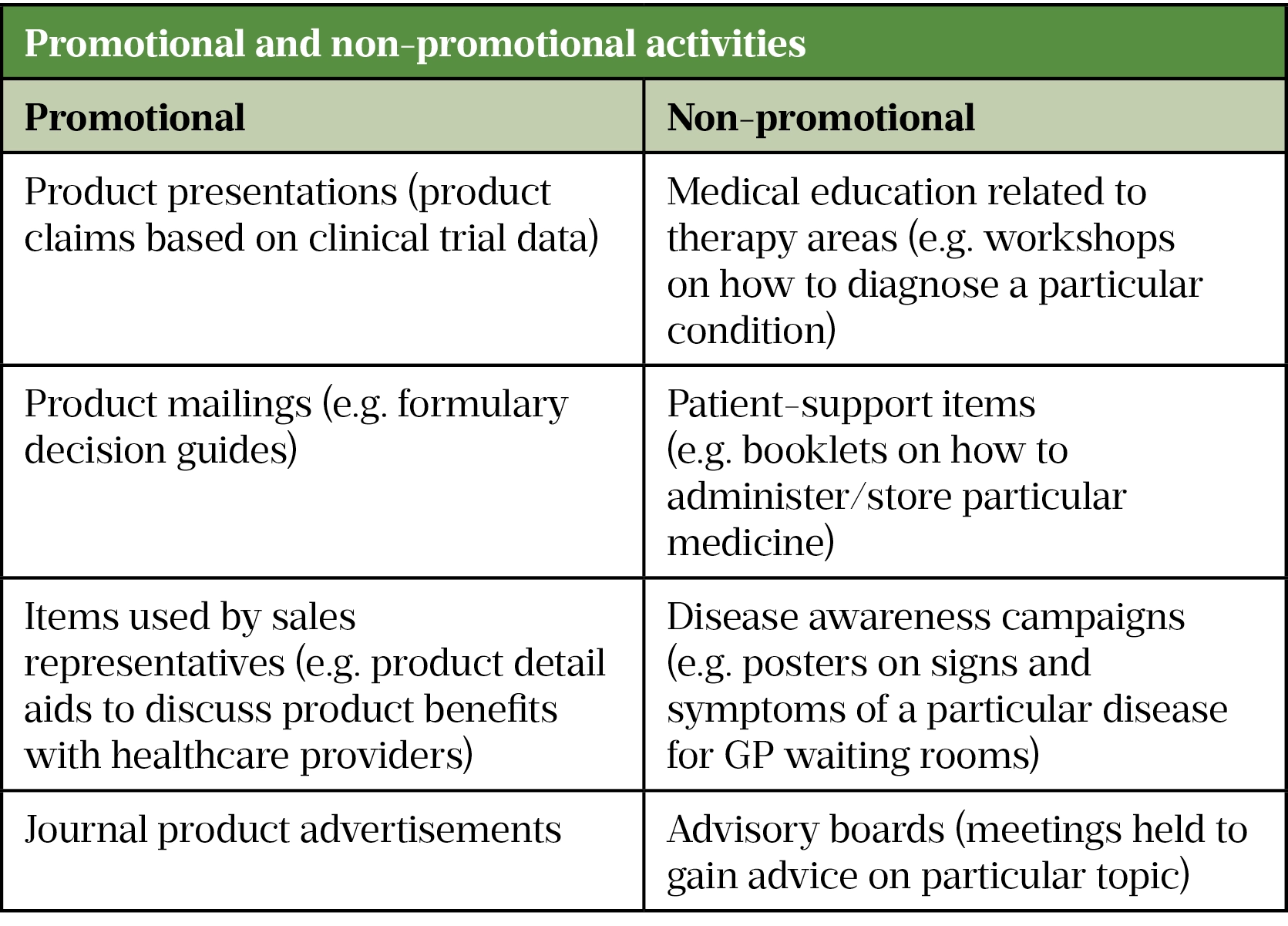
All promotional material (i.e. material associated with a product) and certain non-promotional material (i.e. does not tend to involve a product) must be approved by a final medical signatory. Some examples of promotional and non-promotional activities are shown in Table 1.
Objectives of the role
Final medical signatories ensure promotional materials and activities are fair, balanced and factually accurate. Materials are often tailored to insights gathered by industry from both patients and healthcare professionals. Although medical signatories are not patient facing, their function contributes to informed prescribing decisions influencing standard of patient care.
Pharmacists therefore possess many of the skills relevant for this role. As medicines experts, they have direct exposure to patients and other healthcare professionals, and have a wide breadth of understanding and knowledge of different disease areas alongside scientific knowledge.
Initially, pharmacist signatories were only permitted to provide final approval of materials in selected areas of the APBI Code of Practice under supervision by qualified medical signatory doctors. However, since 2014 pharmacist signatories can now cover all parts of the code without supervision.
Many opportunities are available to pharmacist final medical signatories within the pharmaceutical industry: roles in medical affairs; marketing and compliance; global and affiliate-level prospects; bespoke review and approval roles within medical compliance agencies working in collaboration with the industry; and many more. Some medical signatories also choose to undertake contracting or consultancy roles for different organisations.
Responsibilities within an organisation
The ABPI Code of Practice is developed and revised periodically, using various sources. These include European and international codes of practice, regular reviews, UK-specific legislation and consultations with various stakeholders, including pharmaceutical companies. The 2021 ABPI Code of Practice sets out 31 clauses (each with sub-clauses) of clear guidance on the criteria that need to be met[1]. The final medical signatory needs to be cognisant of all parts of the Code to allow for appropriate guidance and approval of specific materials depending on their purpose and objective. An example of an interesting criteria is that the cost of any subsistence (food and drink) provided by a company to a delegate at a UK meeting must not exceed £75 per person, excluding VAT and gratuities.
International activities conducted by a UK-based company also require a final medical signatory to be aware of the requirements of other codes of practice[1] (for example, an activity carried out by a UK company in Spain would need the final medical signatory to ensure compliance with both the Spanish and UK codes of practice). This multi-country regulation awareness often means UK final medical signatories have to collaborate with colleagues around the world.
Senior signatories provide mentorship and training to new signatories, are involved in the complaints process where a company is alleged to have breached the ABPI Code of Practice, represent organisations at hearings and share best practice in review and approval across wider business units.
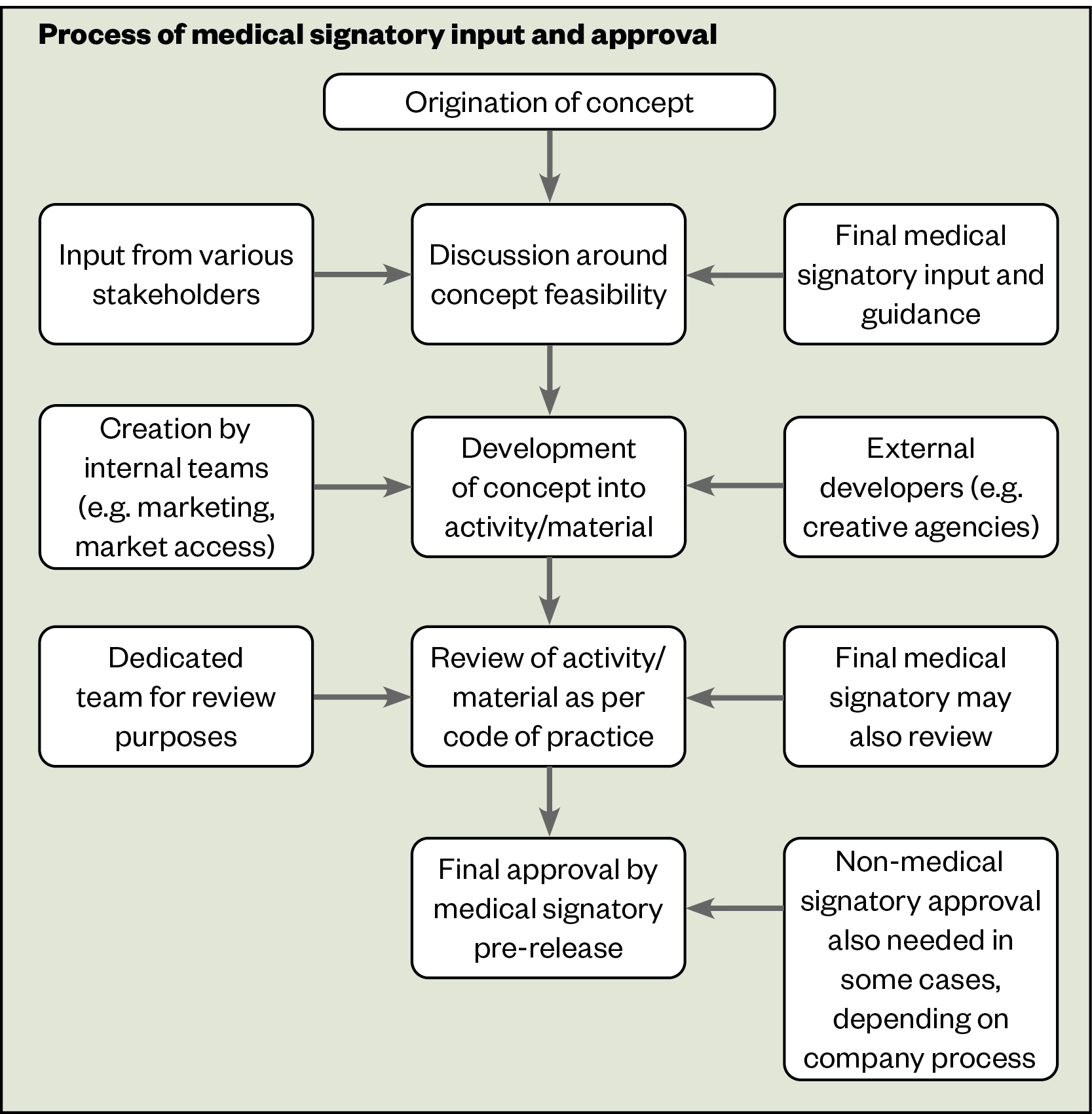
Figure 1 shows a flowchart of final signatory involvement in the approval process.
Non-compliance can lead to breaches of the ABPI Code of Practice, with wide-ranging sanctions for the responsible company: a public reprimand, recovery of material from recipients, financial sanctions, audit and, in some instances, pre-vetting of materials[1]. In cases of serious breaches, advertisements naming companies that breach the Code are placed in the pharmaceutical, medical and nursing press. The final medical signatory (who is ultimately accountable) could also face personal liability. The MHRA, which administers UK law, can intervene if there are serious public health concerns or the model of self-regulation fails. Further training or re-assessment for the signatory could be an intervention as part of the learning process.
Route to becoming a final medical signatory
There are different routes to becoming a final medical signatory that encompass specific skills and training. The training and requirements are customised, depending on the organisation offering the role.
A pharmacist must be registered with the General Pharmaceutical Council to become a final medical signatory. The required experience level will vary depending on the pharmaceutical company, but some industry experience will be necessary in most instances. Other important considerations include experience in previous clinical roles, as well as product and therapy area knowledge.
Skills, knowledge and experience required
Final medical signatories must develop a variety of skills and knowledge to thrive within the role. These include teamwork, communication, technical knowledge and research skills. Many of these skills can be developed while working as a clinical pharmacist.
Cross team work
Strong cross-functional partnership working with other teams, such as marketing, regulatory, market access, training and compliance departments is a core requirement. The ability to communicate with commercial and non-commercial teams, both written and verbal, is often important to ensure alignment across the various functions to have a smooth compliance journey.
Pharmacists currently working in clinical practice can look to develop this transferable skill through active participation in multidisciplinary team working to ultimately benefit patients.
Good time management, balancing conflicting priorities and integrating within large teams by providing specific specialist support are all important skills to develop and can be worked on at any time.
Attention to detail
Technical understanding and implementation of the ABPI Code of Practice requires attention to detail. In some ways, final medical approval is similar to ensuring clinical accuracy when assessing a drug chart or clinically checking a prescription.
Learning modules during signatory training often focus on each clause of the Code in specific detail.
Knowledge in multiple disease areas
Having knowledge across multiple disease areas is often advantageous, depending on the therapy area that a pharmaceutical company specialises in. This is often developed by pharmacists during the MPharm and foundation level training. Individuals should look to supplement this knowledge through self-study, post-graduate and prescribing courses, and rotation through as many clinical areas as possible as a practicing pharmacist.
Training on a particular disease area is often provided by pharmaceutical companies, depending on the area of that company’s interest.
Focusing on solutions
The ability to offer solutions when challenges arise is vital. In clinical practice, providing alternative pharmacological options to inappropriate therapy is an example of how pharmacists can practice this skill.
Interprofessional skills
Interprofessional skills are crucial to demonstrate ethical leadership when working with a diverse set of individuals and adhering to the strict requirements for final approval.
Training and registration
In the absence of any formal requirements, each pharmaceutical company will have its own standards and training to become a final signatory. This is often blended learning and includes:
- In-house training where the company organises an individual package of training;
- External training by an agency;
- Mixture of in-house and outsourced training;
- Mentorship by senior signatories within the organisation;
- Reviews of past precedents;
- Shadowing a qualified final signatory;
- Product-specific training, including wider data set.
Companies have standard operating procedures that are built from the principles of the ABPI Code of Practice and these play a crucial role in the training of final signatories. A thorough understanding of internal processes is needed to confidently meet the external requirements.
The length of training varies and again will be down to the individual company. There will often be some form of assessment at the end of the training period. Upon successful completion of the training and assessment, names of nominated final medical signatories are submitted by the company to the PMCPA and MHRA.
Where to find opportunities
Often, final-signatory opportunities are provided as part of another industry role, for example within a medical affairs position, and balanced alongside other responsibilities, such as providing strategic input, project management and healthcare professional engagement on a day-to-day basis. However, full time signatory roles do exist. It is important when searching for roles to look broadly at opportunities and where they can lead. Companies will often look for foundation-level knowledge and understanding of the pharmaceutical industry before progression into final medical signatory training, but this is not always necessary.
Not understanding the role in detail will weaken a candidate’s application so networking with those already in the role to gain better insight can often be fruitful. LinkedIn is a great professional networking space to begin relationships and for knowledge sharing, and Indeed is a wide-ranging job platform that is updated on a regular basis with clear requirements.
Pharmacists working in a different sector should use their current role to enrich their skillset wherever possible. This will prepare individuals for a transition to final medical signatory when the opportunity arises.
- 1ABPI Code of Practice for the Pharmaceutical Industry 2021. 2021. https://www.pmcpa.org.uk/media/3406/2021-abpi-code-of-practice.pdf (accessed Jan 2022).