Shutterstock.com
This article describes the formulation of dosage forms that are designed to be administered topically to the eye (principally the conjunctiva or the eyelid) for the treatment of primarily local disorders. Glaucoma, a disease of the interior of the eye, may also be treated using these dosage forms.
Three main types of dosage form are clinically used:
- Solutions
- Suspensions
- Ointments.
The administration of these treatments to the eye is usually performed using a dropper (or a container with a dropper nozzle) or a tube with a nozzle. Access of principally solution or suspension formulations to the remote anatomical regions of the eye may also be achieved parenterally (termed intraocular injections) to the anterior, posterior and vitreous chambers (Figure 1). Ocular formulations (akin to parenteral formulations) must be sterile.
Advantages and disadvantages of ocular dosage forms
Advantages
- The application of the therapeutic agents directly to the site of action ensures that the therapeutic agent is available at higher concentrations than may be achieved following oral administration.
- Administration of the therapeutic agent locally ensures that the incidence of side effects is minimised.
- Following training, the administration of the dosage form locally to the eye may be easily performed by the patient.
Disadvantages
- The retention of the drug at the site of action is relatively poor, due principally to the low tear volume (7µl for the blinking eye, 30µl for the non-blinking eye). The typical volume of two drops of a solution formulation is circa 100µl and therefore the majority of the applied dose is lost either through spillage on to the face or via the lacrimal duct.
- In addition, the retention time of applied solutions on the surface of the eye is poor. For example, it has been reported that, following the administration of a pilocarpine solution to the eye, removal of the solution from the precorneal region of the eye occurs in less than 2 minutes, resulting in absorption of approximately 1% of the originally applied dose of drug.
- Therefore, to overcome these deficiencies in practice, the patient is required to administer the ocular solution formulations (containing high concentrations of therapeutic agent) frequently, which is inconvenient and may lead to patient non-compliance. These inadequacies have inspired pharmaceutical scientists to devise strategies by which the retention of the drug within the precorneal region may be enhanced.
- Ocular formulations are sterile and therefore specialist facilities are required for the manufacture of these dosage forms.
- Local side effects may be experienced to ocular dosage forms (to either the high concentration of therapeutic agent (≤5% w/w) or excipients used in the formulation). Typically pain and irritation are the major side effects encountered by patients.
- The application of ointment formulations to the eye may result in a temporary blurring of vision.
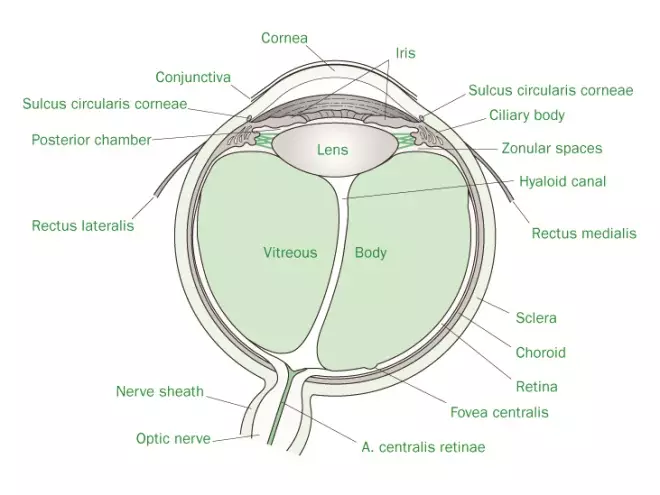
Source: Pharmaceutical Press
Figure 1: Cross-section of the anatomy of the eye
Administration of therapeutic agents to the eye
The main anatomical features of the eye are illustrated in Figure 1.
The main features of the eye that are of interest for ocular drug delivery and hence the formulation of ocular dosage forms are:
- Conjunctiva
- Cornea
- Lacrimal fluid.
Conjunctiva
- The conjunctiva is located at the side of the eye and joins on to the cornea and eyelids.
- The surface area of the conjunctiva is large (circa 18 cm).
- The conjunctiva helps produce and maintain the tear film.
- The permeability of the conjunctiva to the diffusion of therapeutic agents is greater than that of the cornea.
Cornea
- The cornea is composed of three layers:
- epithelium (adjacent to the conjunctiva): a multilayered epithelium that is rich in lipids
- stroma (central region): this is an aqueous matrix composed of collagen and keratocytes
- endothelium: a lipid-rich, single-cellular epithelium that maintains corneal hydration.
- The diffusion of drugs into the inner chambers of the eye is controlled by the cornea; diffusion occurs via paracellular routes.
- The lipid outer and inner layers (epithelium/endothelium) of the cornea and the predominantly aqueous stroma control drug diffusion into the internal regions of the eye. To be effectively absorbed, therapeutic agents must exhibit intermediate solubility in both these lipid and aqueous phases and must be of low molecular weight.
- The cornea is non-vascular and negatively charged.
Lacrimal fluid
- Lacrimal fluid is secreted from glands and is located on the surface of the eye.
- The pH of the lacrimal fluid is 7.4 and this fluid possesses a good buffer capacity (due to the presence of carbonic acid, weak organic acids and protein), being able to neutralise unbuffered formulations effectively over a wide range of pH values (3.5–10.0).
- Lacrimal fluid is isotonic with blood. Typically ocular aqueous dosage forms are not specifically formulated to be isotonic (0.9% w/w NaCl equivalent) and may be formulated within a range of tonicity values equivalent to between 0.7% and 1.5% w/w NaCl.
- The rate of turnover of lacrimal fluid is approximately 1 µl/min and the blinking frequency in humans is circa 15–20 times per minute. These physiological functions act to remove the therapeutic agent/formulation from the surface of the eye.
Formulation considerations for aqueous ocular dosage forms
There are two categories of aqueous ocular dosage forms:
- Ocular solutions
- Ocular suspensions.
While the vast majority of aqueous ocular dosage forms are solutions, suspensions may be required whenever the therapeutic agent exhibits problems regarding chemical stability or, in the case of steroids (e.g. dexamethasone, prednisolone), the potency of the lipophilic drugs is greater than that of the water-soluble salts. The majority of formulations considerations for ocular dosage forms are similar to those described for pharmaceutical solutions in general. Accordingly the main considerations for the formulation of ocular solutions and suspensions are as follows.
Choice of drug salt for use in ocular solutions
One of the main determinants surrounding the choice of salt type for inclusion in solution formulations is the solubility – the salt form being chosen to achieve the required solubility. To compensate for the poor retention of therapeutic agents within the precorneal region (and therefore to achieve the maximum pharmacological effect), the concentration of therapeutic agents in ocular solutions is relatively high. Under these circumstances the choice of drug salt is important as the local ocular application of certain drug salts results in greater pain/irritation (e.g. stinging). This may be exemplified by considering three salt forms of adrenaline (epinephrine) (the hydrochloride, borate and bitartrate salts), their physicochemical properties in solution and the associated pain when applied to the eye. Adrenaline bitartrate is the most acidic salt in solution (pH 3–4) and, due to the strong buffer capacity, has been reported to produce moderate to severe stinging following application. Conversely, only mild stinging is associated with the topical application of the borate salt due to the low buffer capacity and lower acidity of this salt (pH 5.5–7.5). Adrenaline hydrochloride, although relatively acidic (pH 2.5–4.5), exhibits medium buffer capacity and is therefore more effectively neutralised than the bitartrate salt. This results in reduced stinging in comparison to the bitartrate salt. Importantly, as the discomfort of the salt form increases, there will be a greater tendency for the drug to be washed off the surface of the eye due to the production of lacrimal fluid.
Physical properties of the dispersed therapeutic agent
The physical properties of the therapeutic agent are important design features of ophthalmic suspensions and indeed in all types of suspension dosage forms. The particle size used in ocular suspensions is of primary importance due to the relationship between particle size and ocular irritation. Typically 95% of dispersed particles should have an average particle diameter that is less than 10µm.
Solution pH/inclusion of buffers
The pH and the control of the pH of ocular formulations are important determinants of the stability of the therapeutic agent, the ocular acceptability of the formulation and the absorption of the drug across the cornea.
Ideally the pH of the formulation should be that which maximises the chemical stability (and, if required, absorption) of the therapeutic agent. This issue is particularly important due to the effect of pH on the stability of alkaloid drugs, e.g. atropine, pilocarpine, carbochol. As highlighted in the previous section, the pH and the buffer capacity directly affect the subsequent discomfort of the formulation.
| Table 1 The effect of temperature and pH on the stability (half-life) of pilocarpine and atropine | |||
|---|---|---|---|
| Drug | Temperature(°C) | pH | Half-life |
| Atropine | 121 | 6.8 | 1h |
| 121 | 5 | 60 h | |
| 25 | 6.8 | 2 years | |
| 25 | 5 | 130 years | |
| Pilocarpine | 121 | 6.8 | 34 min |
| 121 | 5 | 24 h | |
| 25 | 6.8 | 66 days | |
| 25 | 5 | Several years | |
Ideally the pH of the ocular solution should be controlled at 7.4 as this is the pH of tear fluid. However, the choice of pH of the formulation is also dictated by the stability of the therapeutic agent at that pH (which in turn serves to define the shelf-life of the formulation) and whether (or not) absorption of the active agent across the cornea is required.
Chemical stability
pH is an important contributor to the degradation of therapeutic agents that undergo hydrolysis, e.g. alkaloids. The effect of pH on the stability of two alkaloids at two different temperatures is shown in Table 1.
As may be observed, the stability of the two alkaloids at room temperature increases as the pH is increased. Therefore, solutions of these alkaloids are prepared at the lower pH to ensure that the product has a commercially viable shelf-life. Interestingly, exposure to high temperatures, such as those encountered during moist-heat sterilisation, also reduces the stability of these therapeutic agents (although this is significantly less within a formulation of lower pH). Therefore, the choice of pH may also affect the method by which the formulation is sterilised. Importantly, if the pH of alkaloidal solutions is buffered to pH 6.8 (e.g. using a phosphate buffer), the formulations must not be sterilised using thermal methods. For this purpose aseptic preparation involving sterile filtration is used.
Drug absorption across the cornea
The successful treatment of glaucoma using ocular formulations requires that there is sufficient drug absorption across the cornea. To be effectively absorbed the drug must exhibit differential solubility, i.e. the ionised and non-ionised forms coexist. The effects of pH and ionisation on the absorption of drugs across the cornea are represented in Figure 2.
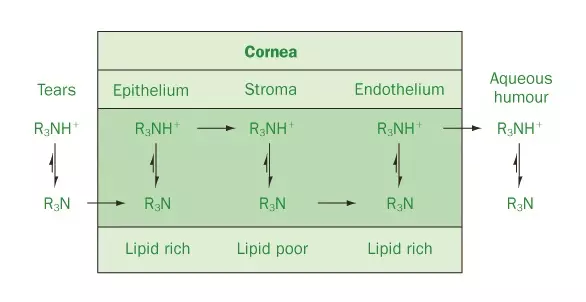
Source: Pharmaceutical Press
Figure 2: Diagrammatic representation of the effect of ionisation (and hence pH) on drug absorption across the cornea
As may be observed, sufficient concentration of the non-ionised form is required to partition into and diffuse across the lipid-rich outer layer of the cornea (the epithelium). The inner layer of the cornea (the stroma) is predominantly aqueous and therefore ionisation of the drug must occur to enable partitioning into this phase. Following diffusion to the interface of between the stroma and the endothelial (lipid-rich) layer, absorption of the non-ionised (but not the ionised) form occurs. The non-ionised drug then diffuses to the endothelium/aqueous humour interface where ionisation and dissolution into the aqueous humour occur. The pKa of the therapeutic agent determines the ionisation of the therapeutic agent at defined pH values. The role of pH on the stability of alkaloids was highlighted in the previous section. Therefore, whilst the chemical stability of alkaloids is enhanced in formulations of lower pH (5), under these conditions the therapeutic agent will be predominantly ionised (circa 99%) and drug absorption will be poor. Increasing the pH of the formulation will decrease the percentage ionisation of the therapeutic agent (and hence increase absorption); however, the chemical stability may be compromised.
To overcome this problem alkaloidal drugs are frequently used in the form of the acid salt, where the pH of the solution will be acidic and stability is optimised. No buffers are added and therefore whenever the formulation is instilled into the eye, the lacrimal fluid adjusts the pH to physiological conditions, thereby facilitating absorption.
Tip
Most eye drop formulations are solutions due to the less complex nature of the formulation (as compared to suspensions) and greater comfort in use.
If pH control is required, e.g. for acidic drugs, a borate buffer may be used to produce solutions of acidic pH (circa 5). Stabilisation of this pH within the lacrimal fluid will, if required, enhance the absorption of acidic drugs. Phosphate buffers may be used to control the pH of eye drops (at physiological pH). Finally it should be remembered that control of the pH is often used to prevent precipitation of the therapeutic agent in the formulation.
Vehicle
The vehicle that is predominantly used for the formulation of aqueous ocular dosage forms is Purified Water USP. Water for injections is not a specific formulation requirement. Occasionally oil is used if the therapeutic agent is profoundly unstable within an aqueous vehicle. The choice of oils for ocular use is similar to that for parenteral use.
Viscosity-modifying agents
Viscosity-modifying (enhancing) agents are hydrophilic polymers that are added to ocular solutions for two main reasons: (1) to control the rate at which the drop flows out of the container (and thus enhance ease of application); and, more importantly, (2) to control the residence time of the solution within the precorneal environment. For example, it has been shown that the retention of an aqueous solution within the precorneal region is short (frequently less than 1 minute); however, if the viscosity is increased, the retention may be enhanced. Furthermore, it has been reported that there is a critical formulation viscosity threshold (circa 55 mPa/s) above which no further increase in contact time between the dosage form and the eye occurs. It must be remembered that there is an upper limit below which the viscosity of eye drop formulations must be maintained as this may lead to blockage of the lacrimal ducts. The viscosities of commercially available products are frequently less than 30 mPa/s. The enhancement of the viscosity of ocular suspensions will serve to enhance the physical stability of ocular suspensions.
Ideally the viscosity-modifying agent should exhibit the following properties:
- Easily filtered: all eye drop solutions are filtered during the manufacturing process. This may be simply to remove any particles (e.g. clarification using a 0.8-µm filter) or clarification in conjunction with filtration sterilisation.
- Easily sterilised: sterilisation of eye drop solution is usually performed by filtration (see above) or by heat. If heat sterilisation is performed, the viscosity-modifying agent must be chemically and physically stable under these conditions.
- Compatible with other components: the interaction of hydrophilic polymers with certain preservatives is a well-known phenomenon and is usually resolved by increasing the concentration of preservative. However, the pharmaceutical scientist must ensure that there is no or only limited interaction between the therapeutic agent and the viscosity-modifying agent, e.g. basic therapeutic agents and polyacidic polymers.
Examples of polymers that are used as viscosity-modifying agents include:
- Hydroxypropylmethylcellulose (Hypromellose USP). Hydroxypropylmethylcellulose (HPMC) is a partially methylated and O-(2-hydroxypropylated) cellulose derivative. In aqueous ocular formulations HPMC is used in the concentration of 0.45–1.0% w/w.
- Poly(vinyl alcohol). This is a water-soluble vinyl polymer that is available in three grades: (1) high-viscosity (average molecular weight 200 000 g/mol); (2) medium-viscosity (average molecular weight 130 000 g/mol); and (3) low-viscosity (average molecular weight 20 000 g/mol). It is used to enhance the viscosity of ocular formulations in concentrations ranging from 0.25% to 3.00% w/w (the actual concentration being dependent on the molecular weight of polymer used).
- Poly(acrylic acid). This is a water-soluble acrylate polymer that is cross-linked with either allyl sucrose or allyl ethers of pentaerythritol. It is predominantly used in ocular aqueous formulations for the treatment of dry-eye syndrome. However, it may be used to increase the viscosity of ocular formulations that contain a therapeutic agent.
Preservatives
Ocular formulations are sterile; however, as they are designed as multidose systems, the addition of a preservative is required. This section will specifically address issues regarding the preservation of ophthalmic formulations, including a description of the key preservatives that are used in this class of pharmaceutical product.
Cationic preservatives
The two main cationic preservatives that are commonly used in ocular solutions and suspensions are benzalkonium chloride and benzethonium chloride. It should be noted that cationic preservatives are incompatible with anionic therapeutic agents and anionic agents that are used for the control (e.g. pilocarpine nitrate, physostigmine) or diagnosis (e.g. sodium fluorescein) of ocular conditions. Furthermore, interaction of these compounds may occur with non-ionic hydrophilic polymers (used to modify the viscosity of ocular formulations).
Benzalkonium chloride
This is a mixture of alkylbenzyldimethylammonium chlorides that is used in ocular solutions/suspensions at a concentration between 0.002% and 0.02% w/v (typically 0.01% w/v). The resistance of certain microorganisms that are ocular pathogens to benzalkonium chloride (most notably Pseudomonas aeruginosa) has been observed. Therefore, it is customary to include 0.1% w/v disodium edetate (disodium EDTA) in ocular formulations in which benzalkonium chloride is used. This agent acts to enhance the antimicrobial activity of benzalkonium chloride by chelating divalent cations in the outer membrane of the bacterial cell, thereby rendering the bacteria more permeable to the diffusion of the antimicrobial agent. The antimicrobial properties of benzalkonium chloride decrease whenever the pH of the formulation falls below 5.
Benzethonium chloride
Benzethonium chloride (unlike benzalkonium chloride) is a pure compound (and not a mixture of compounds). It is commonly used in ophthalmic formulations within the concentration range 0.01–0.02% w/v (although it has been reported to exhibit lower antimicrobial activity than benzalkonium chloride).
Esters of parahydroxybenzoates (parabens)
Mixtures of methyl and propyl esters of parahydroxybenzoic acid are used in ophthalmic formulations (typically at a combined concentration of 0.2% w/w). There is a concern regarding the ocular irritancy of the parabens, which limits their use in ophthalmic preparations. This problem is augmented by the need to increase the concentration of parabens in ocular formulations that contain hydrophilic polymers (due to the interaction between these two species).
Organic mercurial compounds
These are antimicrobial agents that contain mercury and, due to environmental and toxicity concerns, are not commonly used in ocular formulations nowadays. The main examples of these compounds that have found pharmaceutical use are phenylmercuric acetate, phenylmercuric nitrate (which is sometimes supplied as a mixture with phenylmercuric hydroxide) and thimerosal. The typical concentration ranges of the antimicrobial agents used in ocular formulations are: 0.001–0.002% w/v for phenylmercuric acetate, 0.002% w/v for phenylmercuric nitrate and 0.001–0.15% w/v and 0.001–0.004% w/v thimerosal (when used in ophthalmic solutions and suspensions, respectively).The phenylmercuric salts have been reported to be deposited in the lens of the eye (termed mercuria lentis) when formulated in preparations that are designed for chronic usage, e.g. for the treatment of glaucoma. Thimerosal is not associated with this problem; however, it has been associated with ocular sensitisation. As a result, these preservatives are only used in ocular formulations whenever there is no suitable option
Organic alcohols
Two main organic alcohols are used as preservatives for ophthalmic formulations: (1) chlorobutanol; and (2) phenylethylalcohol. The main features of these are described below.
Chlorobutanol
Chlorobutanol is used in ocular formulations at a concentration of circa 0.5% w/v. Under alkaline conditions hydrolysis of chlorobutanol occurs, liberating HCl as a by-product (the rate of reaction increases with increasing temperature, e.g. during autoclaving).
Therefore, the use of chlorobutanol is reserved for acidic ophthalmic preparations. In addition, chlorobutanol is volatile and lost from solution if stored in polyolefin containers (due to partitioning). Accordingly, preparations that employ this preservative must be stored in glass containers. One final problem associated with the use of chlorobutanol is its limited solubility. The concentration of chlorobutanol used in ophthalmic preparations (0.5% w/v) is close to the saturation solubility (0.7% w/v at room temperature). Therefore, if the formulation is stored below room temperature, precipitation of the preservative may occur in situ.
Phenylethylalcohol
Phenylethylalcohol has similar properties to chlorobutanol and indeed shares similar problems, e.g. poor solubility, volatility and partitioning into plastic containers. The typical concentration used in ophthalmic preparations is 0.25–0.50% v/v.
Antioxidants
As in other formulations, antioxidants may be added to ocular solutions/suspensions to optimise the stability of therapeutic agents that degrade by oxidation, e.g. epinephrine. Sodium metabisulphite (circa 0.3%) is an example of an antioxidant that is commonly used for this purpose.
Tips
- The use of mercuric preservatives in eye drop formulations is less common than for other preservatives due to possible concerns regarding toxicity.
- Cationic surfactants are commonly used as preservatives in eye drop formulations.
- Eye drop formulations are sterile. The inclusion of preservatives is required due to the multidose nature of this type of dosage form.
Surface-active agents
Surface-active agents are predominantly employed in aqueous suspension to enhance the physical stability of the dispersed particles; however, there have been reports concerning the use of these agents to solubilise therapeutic agents in aqueous ocular solutions. One of the primary concerns regarding the use of surface-active agents in ocular dosage forms is the potential toxicity/irritancy. Accordingly, non-ionic surfactants are preferentially (and predominantly) used whereas the use of anionic surfactants on ocular solution/suspension dosage forms is avoided.
Tip
The formulation of eye drop dosage forms involves the use of similar components to those used in the formulation of conventional solutions; however, it should be remembered that ocular dosage forms are sterile.
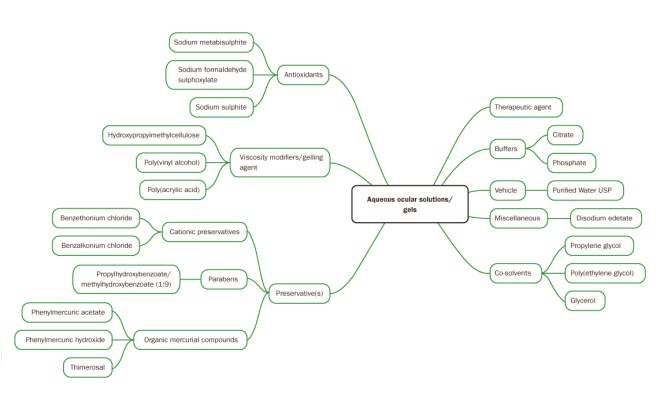
Source: Pharmaceutical Press
Figure 3: Schematic of forumlation considerations for aqueous ocular solutions and gels
A summary of the formulation considerations for aqueous ocular solutions and ocular gels is provided in Figure 3.
Manufacture of aqueous ophthalmic formulations
The manufacture of aqueous ophthalmic solutions/suspensions is similar to that described for the manufacture of parenteral solutions and suspensions. Akin to parenteral formulations, ophthalmic solutions/suspensions must be sterile. The manufacture of these systems may involve at least one of the following steps.
Production under clean conditions followed by sterilisation by autoclaving
Typically ophthalmic solutions may be manufactured and packaged in the final container under clean conditions. Sterilisation may then be performed using moist-heat sterilisation (assuming the therapeutic agent is chemically stabile under these conditions).
Production under clean or aseptic conditions followed by aseptic sterilisation by filtration
If the therapeutic agent is thermolabile then sterilisation by heat should be avoided. The production of ocular solutions is then performed by initially manufacturing the dosage form under clean or aseptic conditions. Clarification of the solution is performed by filtration through an appropriate filter (circa 1µm), followed by sterilisation filtration (0.22µm filter) and packaging (both under aseptic conditions).
Production under aseptic conditions
Ophthalmic suspensions may not be sterilised by filtration. Therefore, the manufacture of these systems usually involves the dispersion of the sterile therapeutic agent into the sterile vehicle (with added excipients) and subsequent packaging, both under aseptic conditions.
Formulation considerations for ophthalmic ointments
Ophthalmic ointments are commonly used for the treatment of ocular infection and/or inflammation. One major disadvantage associated with the use of ophthalmic ointments is their general greasiness and the associated (temporary) blurring of vision. For these reasons patients may prefer aqueous ocular formulations or, alternatively, may prefer to reserve the use of ophthalmic ointments to night-time application. Due to their greater retention within the precorneal region (approximately 2–4 times greater than for aqueous solutions), the frequency of administration of ointments is generally lower than their aqueous-solution counterparts.
As is the case for all ointment formulations, ophthalmic ointments are prepared by dispersion of the therapeutic agent in the pre-prepared ointment base.
Ointment bases for ocular administration
Various types of ointment bases may be employed for ocular administration, as follows.
Hydrocarbon bases
Hydrocarbon bases are widely used in ophthalmic ointments and are generally composed of a mixture of paraffins (to achieve the correct viscosity). In the formulation of these systems, yellow soft paraffin is preferred to white soft paraffin: white soft paraffin is a bleached form of yellow soft paraffin, thereby being associated with a lower potential for ocular irritancy.
Non-emulsified absorption bases
These bases are composed of one or more paraffins (e.g. liquid and yellow soft paraffin) and a sterol containing emulsifying agent (e.g. lanolin derivatives). These bases are less greasy than those prepared using hydrocarbons alone and, in addition, aqueous solutions of drug may be incorporated into non-emulsified absorption bases.
Water-soluble bases/aqueous gels
Water-soluble bases may be successfully administered to the eye and are associated with a lower incidence of blurred vision (due to their water-miscibility). These bases are primarily formulated using polyethylene glycols. More recently aqueous gels containing a dissolved or dispersed therapeutic agent have been successfully used for the treatment of infection and/or inflammation. Aqueous gels composed of poly(acrylic acid) are commonly used for this purpose and, in addition, have been successfully employed for the treatment of glaucoma (requiring fewer administrations per day in comparison to aqueous solutions containing this drug). Furthermore, poly(acrylic acid) gels (devoid of therapeutic agent) have also been used for the treatment of keratoconjunctivitis sicca (dry-eye syndrome).
Excipients in ocular ointments
The nature of the excipients required in ophthalmic ointments is dependent on the nature of the ointment base used in the formulation. Typically hydrocarbon-based ointment bases and bases that are either devoid of or contain lower concentrations of water require no further excipients. Exceptionally the inclusion of non-aqueous antioxidants, e.g. butylated hydroxytoluene, butylated hydroxyanisole, may be required if the therapeutic agent is prone to degradation by oxidation. Conversely, aqueous systems, e.g. poly(acrylic acid) gels (which may contain 95% w/w water), will require the same types of excipients that are commonly employed in aqueous solutions, e.g. preservatives, buffers and water-soluble antioxidants.
Manufacture of ophthalmic ointments
The manufacture of ophthalmic ointments is very similar to that used for the formulation of ointment formulations; however, one important difference is the requirement that ophthalmic ointments must be sterile. Due to their relatively high viscosity, terminal sterilisation of ointments is a difficult operation and may only be performed (in limited examples) at elevated processing temperatures. Therefore, ointments are typically manufactured and packaged under aseptic conditions.
The components of the base are customarily prepared in an enclosed mixing vat to which heat may be applied both to aid dissolution and mixing of the ingredients and, importantly, to sterilise the ointment base. The sterile therapeutic agent may then be added to the sterile base and mixed until homogeneous. Filling of the ointment into the final container is performed under aseptic conditions.
A summary of the formulation considerations for ocular ointments is provided in Figure 4.
Tips
- The formulation of ocular ointments involves the use of similar components to those used in the formulation of conventional ointments; however, it should be remembered that ocular dosage forms are sterile.
- Eye drop formulations are generally preferred to ocular ointments by patients due to the ease of administration and non-blurring of vision associated with eye drops.
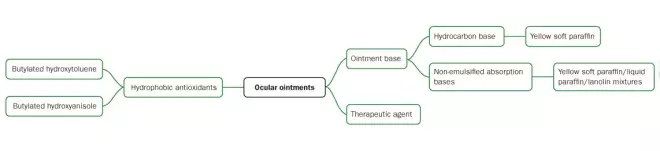
Source: Pharmaceuticall Press
Figure 4: Schematic of formulation considerations for ocular ointments
Quality control of ocular products
Akin to parenteral formulations, ocular products must be sterile and therefore quality control methods must include an assessment of sterility. Therefore, following manufacture and over the designed period of the shelf-life, the following analyses are applied to ocular products:
- Concentration of therapeutic agent: Following manufacture the concentration of therapeutic agent must lie within 95–105% of the nominal concentration. Over the shelf-life of the product the concentration of drug must not fall below 90% of the nominal amount.
- Concentration of preservative: Analysis of preservative concentration within these dosage forms is performed. As before, the concentration of therapeutic agent must lie within 95–105% of the nominal concentration following manufacture and also upon storage.
- Preservative efficacy testing: The efficacy of the preservative(s) within the formulations must be assessed using the appropriate pharmacopoeial method.
- Appearance: All ocular products will have specifications for appearance.
- pH: The pH of aqueous based formulations is measured and compared to the specified range.
- Viscosity: The viscosity of parenteral products is measured and compared to the range defined in the product specifications.
- Dispersed phase size analysis: The particle size distributions of the dispersed drug within suspension formulations are determined using particle size analysis methodology. For example Betaxolol Eye Drops Suspension has limits associated with particle size ranges (not less than 99.5% of particles are less than25 µm, not less than 99.95% are less than 50µm and none exceeds 75µm.
- Freeze–thaw storage: If required, freeze–thaw cycling is performed on the products defined in this article.
- Sterility testing: As described in the introduction to this section, ocular products are required to be sterile, both post-manufacture and over the shelf-life of the product. The assessment of sterility of parenteral products is performed using pharmacopoeial methods (e.g. BP/USP/EP). These methods describe the protocol for the determination of the microbial content within the product, including the composition of the growth media, validation tests and the observation and interpretation of results.
Consult the appropriate pharmacopoeias for a more detailed description of the above methods and others that have not been explicitly covered in this article.
Key points
- Ocular dosage forms are commonly used to treat local ocular disorders, e.g. infection and inflammation; however, intraocular disorders, notably glaucoma, may also be successfully treated.
- Ocular dosage forms are principally solutions, ointments and suspensions. Intraocular injections are available for the treatment of more serious disorders.
- These formulations exhibit similar concerns as comparator formulations regarding physical and chemical stability.
- Ocular dosage forms must be sterile.
- This article was adapted from
FASTtrack: Pharmaceutics – Dosage Form and Design
, published by Pharmaceutical Press.