Centers for Disease Control and Prevention
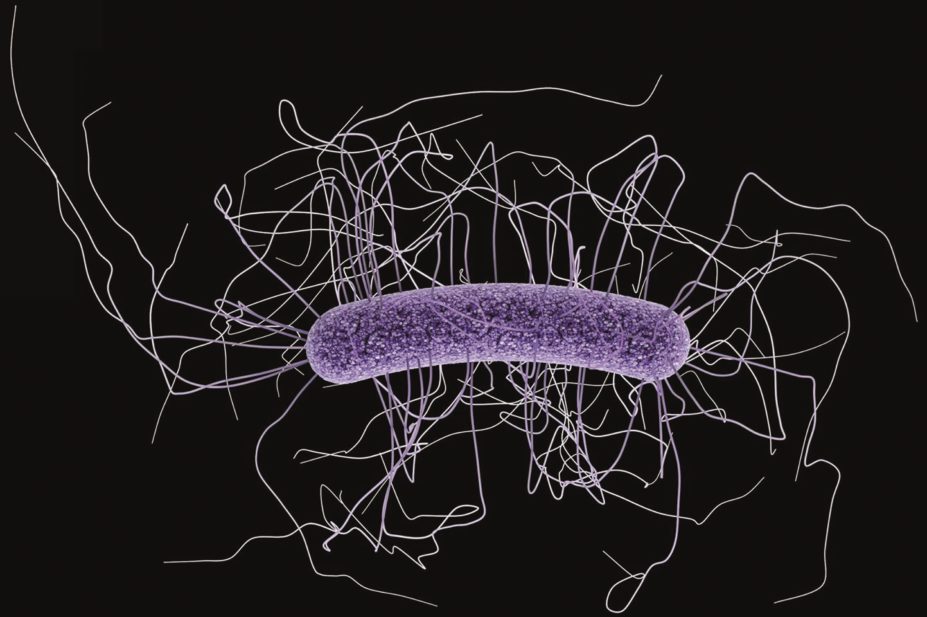
Acid suppression medication is associated with Clostridium difficile infection (CDI) in infants and children aged up to 17 years in an outpatient setting, researchers found in a population-based study, published in Clinical Infectious Diseases on 19 June 2015[1]
.
There has been a ten-fold rise in CDI rates in children from 1991 to 2009, although this shift remains unexplained. One recognised risk factor for CDI in adults is acid suppression medication, a treatment for gastro-oesophageal reflux disease.
Although there has been a dramatic rise in the use of acid suppression medication in children over the past 10–15 years — proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs), most often prescribed — there are few data on acid suppression and CDI risk in paediatric outpatients, and no data on acid suppression and CDI risk in non-hospitalised infants under the age of one.
The study aimed to evaluate the relationship between acid suppression and CDI among infants and children by performing a case-control study nested within a large, outpatient dataset of children aged 0–17 years.
The study found that 2.6% (17 of 650) of children diagnosed with CDI had taken PPIs/H2RAs within 90 days, compared with 0.3% (8 of 3,200) of controls. Using acid suppression medication resulted in an increased risk for CDI: in infants under the age of one (odds ratio [OR], 5.24; 95% confidence interval [CI], 1.13–24.4) and for children aged 1–17 years (OR, 9.33: 95% CI 3.25–26.8). The effect was stronger for PPIs compared with H2RAs.
Senior author Julian Abrams, of New York’s Columbia University Medical Center, and colleagues, believe acid suppression medication may alter the gastrointestinal microbiome and increase the risk of CDI. They note that the gastric flora of children taking H2RAs or PPIs have greater numbers of Streptococcus and other gram-positive bacteria compared with children who do not take acid suppression medication.
Report co-author Daniel Freedberg said: “The microbiome in children is highly mutable up until age four or five. It is unknown whether changes during that formative period, due to antibiotics or PPIs, could alter the microbiome’s ultimate trajectory.”
The researchers looked at data collected from 1995 to 2014 through the Health Improvement Network (THIN), a database maintained by UK general practitioners that holds records of more than 13 million individuals, including electronically captured demographic information, diagnoses and complete prescribing information.
For each case of CDI selected from THIN, up to five eligible control patients were randomly matched, and the control pool comprised those without a diagnosis of CDI at the time when the case had his or her first diagnosis. Use of acid suppression medication with PPIs or H2RAs within 90 days of the index date was defined as the primary exposure, with the prescription issued at least eight days before the index date.
References
[1] Freedberg DE, Lamouse-Smith ES, Lightdale JE et al. Use of acid suppression medication is associated with risk for C. difficile infection in infants and children: a population-base study. Clinical Infectious Diseases 2015. doi:10.1093/cid/civ432.