Source: Shutterstock.com
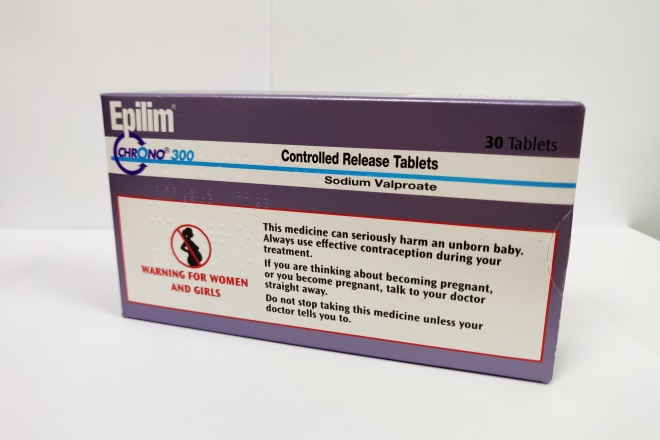
The majority of women prescribed sodium valproate say they are given the patient alert card that warns them of the dangers of taking the drug while pregnant, a widespread NHS audit has found.
Figures from the Pharmacy Quality Scheme (PQS) 2018/2019 valproate audit — which was conducted by just over 90% of community pharmacies in England (10,293) and completed by 94.3% of women taking the epilepsy drug (12,068) — show that 88.9% were given the card by their pharmacist, which is a requirement under Medicines and Healthcare Regulatory Agency (MHRA) guidance introduced in 2018.
Jill Loader, deputy director for pharmacy commissioning in England at NHS England & NHS Improvement, shared the data at the 2021 Pharmacy Show, held in Birmingham on 17–18 October 2021.
The audit data show that almost three quarters (73%) of women prescribed valproate had discussed their medication — and the need for appropriate contraception — with their GP or other specialist in the past 12 months, Loader added.
Of the women surveyed, just 5.6% (675) said they had not been provided with advice and information in line with the MHRA’s 2018 Drug Safety Update, which said that valproate medicines must no longer be used in women or girls of childbearing potential unless a pregnancy prevention programme was in place.
Sodium valproate is a treatment for epilepsy and bipolar disorder, and is prescribed to thousands of women each year. Although prescribing of the drug during pregnancy has fallen by 78% since 2010, MHRA figures show that there was still exposure in 2.46 per 10,000 pregnancies in 2019, which introduces a risk of birth defects in the unborn child.
In 2018, the MHRA changed licensing rules around sodium valproate to minimise these risks by taking measures such as reducing pack sizes and adding warning labels. Under the guidance, pharmacy teams are required to discuss the risks of pregnancy with female patients and issue a warning card each time they dispense these medicines.
A valproate audit published by the Company Chemists’ Association, published in November 2019, found that only 40.1% of community pharmacy teams were issuing the cards when dispensing valproate. This audit was voluntary and was completed before some safety materials were available.
Janice Perkins, former chair of the Community Pharmacy Patient Safety Group, said: “The NHSE results highlight the significant work that community pharmacy teams have done to raise awareness of the risks with patients, families and carers. They have also worked collaboratively with other healthcare professionals to ensure that the MHRA guidance regarding risk assessments etc has been completed and that patient friendly information is shared.
“There’s still more to do and the key challenge is ensuring that the focus on valproate safety becomes part of everyday professional practice and that no patient slips through the safety net.”
Read more: Pharmacy needs to do much better on valproate.