Shutterstock.com
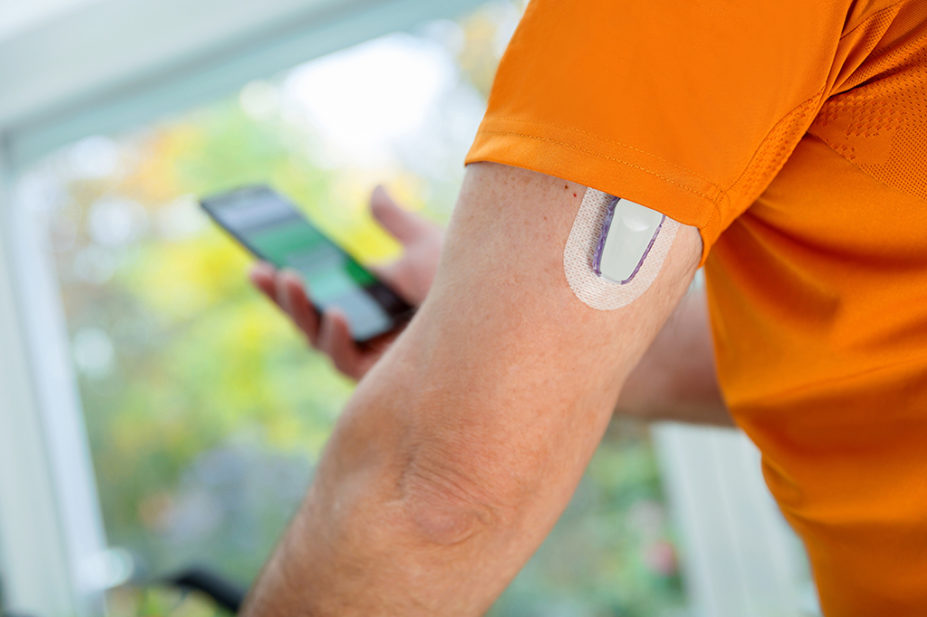
Patients with type 2 diabetes mellitus (T2DM) may be able to benefit from ‘artificial pancreas’ devices that automatically adjust insulin levels based on glucose monitoring and insulin pumps.
A small trial, published in Nature Medicine on 11 January 2023, showed that the closed-loop, fully automated system improved glucose control for 26 patients with T2DM in a 16-week crossover trial, compared to standard insulin therapy.
Some 28 people with T2DM, who use insulin, were recruited to the trial via Addenbrooke’s hospital in Cambridge and local GPs. Two people dropped out before the trial started. Patients spent eight weeks on one type of insulin therapy, then switched to the other for eight weeks, with a two-to-four week washout period between the two.
The study found that people spent more time in the target glucose zone (66.3% of time on average) when on the closed-loop system than standard insulin therapy (32.3% of time on average); HbA1c levels were lower following 8 weeks of using the closed-loop system (7.3%) than standard insulin therapy (8.7%); and time in hypoglycaemia (below 3.9mmol/L) was similar between treatments, with no severe hypoglycaemic events.
During the trial, one serious adverse event occurred — an abscess at the cannula insertion site for the closed-loop system.
One participant withdrew from the study because of inability to manage the devices, but all other participants were able to manage them independently and spent less time managing their diabetes using the closed-loop system.
However, the researchers noted that, as few people use glucose monitors while on standard insulin therapy, it was possible that some of the improved glucose control was a result of glucose monitoring rather than the closed-loop system itself.
The trial was published days after the National Institute for Health and Care Excellence (NICE) published draft guidance outlining that the ‘artificial pancreas’ system should be made available to people with type 1 diabetes who are unable to control their condition through other methods and who have an average HbA1c level of 8% or above, or are pregnant or planning a pregnancy.
The charity Diabetes UK described the draft guidance as “a positive step towards making this life-changing technology available for many more people with type 1 diabetes”.
Nominate an early-careers researcher today for the OPERA23 award
Help us in our search for outstanding early-career researchers who are accomplishing great things in pharmacy and pharmaceutical science.
For entry criteria and details on how to nominate a colleague, click here.